HIV 2-LTR experiment design optimization
- PMID: 30408070
- PMCID: PMC6224063
- DOI: 10.1371/journal.pone.0206700
HIV 2-LTR experiment design optimization
Abstract
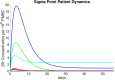
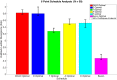
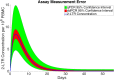
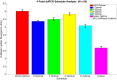
Clinical trials are necessary in order to develop treatments for diseases; however, they can often be costly, time consuming, and demanding to the patients. This paper summarizes several common methods used for optimal design that can be used to address these issues. In addition, we introduce a novel method for optimizing experiment designs applied to HIV 2-LTR clinical trials. Our method employs Bayesian techniques to optimize the experiment outcome by maximizing the Expected Kullback-Leibler Divergence (EKLD) between the a priori knowledge of system parameters before the experiment and the a posteriori knowledge of the system parameters after the experiment. We show that our method is robust and performs equally well if not better than traditional optimal experiment design techniques.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: RZ is named as an inventor on US Patent 9874563, “Detecting and quantifying cryptic HIV replication.” RZ provides consulting to Merck, Sharp, and Dohme, Corp. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Emery AF, Nenarokomov AV. Optimal experiment design. Measurement Science and Technology. 1998;9: 864–876. 10.1088/0957-0233/9/6/003 - DOI
-
- Chaloner K, Verdinelli I. Bayesian experimental design: A review. Statistical Science. 1995;10: 273–304. 10.1214/ss/1177009939 - DOI
-
- Bankert EA, Amdur RJ. Institutional Review Board: Management and Function. Jones & Bartlett Learning; 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases