Characterization of sensory neuronal subtypes innervating mouse tongue
- PMID: 30408082
- PMCID: PMC6224080
- DOI: 10.1371/journal.pone.0207069
Characterization of sensory neuronal subtypes innervating mouse tongue
Abstract
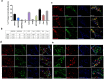
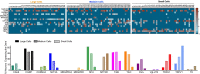
The tongue is uniquely exposed to water-soluble environmental chemicals that may lead to injury or tumorigenesis. However, comparatively little research has focused on the molecular and functional organization of trigeminal ganglia (TG) afferent neurons innervating the tongue. The current study identified and characterized lingual sensory neurons based on a neuronal subtype classification previously characterized in the dorsal root ganglion (DRG) neurons. We employed immunohistochemistry on transgenic reporter mouse lines as well as single-cell PCR of known markers of neuronal subtypes to characterize neuronal subtypes innervating the tongue. Markers expressed in retrogradely labeled TG neurons were evaluated for the proportion of neurons expressing each marker, intensity of expression, and overlapping genes. We found that tongue-innervating sensory neurons primarily expressed CGRP, TRPV1, TrkC, 5HT3A and Parvalbumin. These markers correspond to peptidergic and a subgroup of non-peptidergic C-nociceptors, peptidergic A nociceptors, proprioceptors and myelinated low-threshold mechanoreceptors (LTMRs). Interestingly, as reported previously, we also found several differences between TG and DRG neurons indicating the need for single-cell sequencing of neuronal types based on tissue type within all TG as well as DRG neurons.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Characteristics of sensory neuronal groups in CGRP-cre-ER reporter mice: Comparison to Nav1.8-cre, TRPV1-cre and TRPV1-GFP mouse lines.PLoS One. 2018 Jun 4;13(6):e0198601. doi: 10.1371/journal.pone.0198601. eCollection 2018. PLoS One. 2018. PMID: 29864146 Free PMC article.
-
Characteristics of sensory DRG neurons innervating the wrist joint in rats.Eur J Pain. 2007 Apr;11(3):323-8. doi: 10.1016/j.ejpain.2006.05.003. Epub 2006 Jun 27. Eur J Pain. 2007. PMID: 16807014
-
Calcitonin gene-related peptide immunoreactive neurons innervating the soft palate, the root of tongue, and the pharynx in the superior glossopharyngeal ganglion of the rat.J Chem Neuroanat. 2010 Jul;39(4):221-7. doi: 10.1016/j.jchemneu.2009.12.003. Epub 2009 Dec 23. J Chem Neuroanat. 2010. PMID: 20034556
-
Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia.Cell Tissue Res. 2009 Jun;336(3):349-84. doi: 10.1007/s00441-009-0784-z. Epub 2009 Apr 23. Cell Tissue Res. 2009. PMID: 19387688 Review.
-
Influencers in the Somatosensory System: Extrinsic Control of Sensory Neuron Phenotypes.Neuroscientist. 2023 Aug;29(4):472-487. doi: 10.1177/10738584221074350. Epub 2022 Feb 14. Neuroscientist. 2023. PMID: 35164585 Review.
Cited by
-
Neural substrates of cold nociception in Drosophila larva.bioRxiv [Preprint]. 2025 Jan 27:2023.07.31.551339. doi: 10.1101/2023.07.31.551339. bioRxiv. 2025. Update in: Elife. 2025 Jun 13;12:RP91582. doi: 10.7554/eLife.91582. PMID: 37577520 Free PMC article. Updated. Preprint.
-
Neural substrates of cold nociception in Drosophila larva.Elife. 2025 Jun 13;12:RP91582. doi: 10.7554/eLife.91582. Elife. 2025. PMID: 40512662 Free PMC article.
-
Sex-dependent differences in the genomic profile of lingual sensory neurons in naïve and tongue-tumor bearing mice.Sci Rep. 2023 Aug 12;13(1):13117. doi: 10.1038/s41598-023-40380-6. Sci Rep. 2023. PMID: 37573456 Free PMC article.
-
A pilot study to improve pain phenotyping in head and neck cancer patients.Front Pain Res (Lausanne). 2023 May 11;4:1146667. doi: 10.3389/fpain.2023.1146667. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37251594 Free PMC article.
-
Sensory innervation of masseter, temporal and lateral pterygoid muscles in common marmosets.Sci Rep. 2023 Dec 27;13(1):23062. doi: 10.1038/s41598-023-49882-9. Sci Rep. 2023. PMID: 38155190 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

