Low mucosal-associated invariant T-cell number in peripheral blood of patients with immune thrombocytopenia and their response to prednisolone
- PMID: 30408105
- PMCID: PMC6224073
- DOI: 10.1371/journal.pone.0207149
Low mucosal-associated invariant T-cell number in peripheral blood of patients with immune thrombocytopenia and their response to prednisolone
Abstract
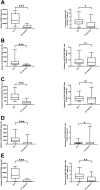
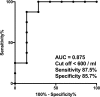
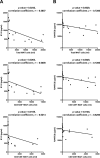
Mucosal-associated invariant T (MAIT) cells help protect against certain infections and are related to some autoimmune diseases. Immune thrombocytopenia (ITP) is a relatively rare hematological autoimmune disease associated with low platelet count. We designed a cross-sectional study wherein we examined peripheral blood samples of patients with ITP for the number of MAIT cells (CD3+TCR-Vα7.2+CD161+IL-18Rα+ lymphocytes) and their CD4/8 subsets (by flow cytometry) and levels of cytokines (by multiplex assays). The study cohort included 18 patients with ITP and 20 healthy controls (HCs). We first compared the number of MAIT cells between HCs and patients with ITP and then performed subgroup analysis in patients with ITP. The number of total MAIT cells in patients with ITP was significantly lower than that in HCs (p < 0.0001), and the CD4-CD8+ subset of MAIT cells showed the same trend. Moreover, patients with ITP refractory to prednisolone exhibited a significantly lower number of total MAIT and CD4-CD8+ MAIT cells than patients sensitive to prednisolone. The number of total MAIT and CD4-CD8+ MAIT cells was not correlated with the response to thrombopoietin receptor agonist treatment or with Helicobacter pylori infection. We found no relation between cytokine levels and response to prednisolone treatment, although the levels of IP-10 and RANTES showed a correlation with the number of total MAIT and CD4-CD8+ MAIT cells. In conclusion, total MAIT and CD4-CD8+ MAIT cells in peripheral blood were decreased in patients with ITP, correlating with their response to prednisolone.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
CD4+/CD8+ mucosa-associated invariant T cells foster the development of endometriosis: a pilot study.Reprod Biol Endocrinol. 2019 Oct 15;17(1):78. doi: 10.1186/s12958-019-0524-5. Reprod Biol Endocrinol. 2019. PMID: 31615517 Free PMC article.
-
[A study of immunocyte subsets and serum cytokine profiles before and after immunal suppression treatment in patients with immune thrombocytopenia].Zhonghua Nei Ke Za Zhi. 2016 Feb;55(2):111-5. doi: 10.3760/cma.j.issn.0578-1426.2016.02.009. Zhonghua Nei Ke Za Zhi. 2016. PMID: 26875579 Chinese.
-
Immune Status and Chemokine C Receptor 7 Expression in Primary in Patients with Immune Thrombocytopenia.Turk J Haematol. 2022 Feb 23;39(1):29-37. doi: 10.4274/tjh.galenos.2021.2021.0281. Epub 2021 Aug 27. Turk J Haematol. 2022. PMID: 34445858 Free PMC article.
-
Functional role of mucosal-associated invariant T cells in HIV infection.J Leukoc Biol. 2016 Aug;100(2):305-14. doi: 10.1189/jlb.4RU0216-084R. Epub 2016 Jun 2. J Leukoc Biol. 2016. PMID: 27256572 Free PMC article. Review.
-
Revealing the protective and pathogenic potential of MAIT cells.Mol Immunol. 2018 Nov;103:46-54. doi: 10.1016/j.molimm.2018.08.022. Epub 2018 Sep 6. Mol Immunol. 2018. PMID: 30196233 Review.
Cited by
-
The biology and functional importance of MAIT cells.Nat Immunol. 2019 Sep;20(9):1110-1128. doi: 10.1038/s41590-019-0444-8. Epub 2019 Aug 12. Nat Immunol. 2019. PMID: 31406380 Review.
-
CpG can induce the proliferation and differentiation of B10 cells in the peripheral blood of children with immune thrombocytopenic purpura.Ann Hematol. 2025 May;104(5):2671-2681. doi: 10.1007/s00277-024-06105-z. Epub 2024 Nov 30. Ann Hematol. 2025. PMID: 39614925 Free PMC article.
-
Immune characteristics and prognostic implications of mucosal-associated invariant T cells in acute myeloid leukemia.Cancer Immunol Immunother. 2023 Dec;72(12):4399-4414. doi: 10.1007/s00262-023-03574-5. Epub 2023 Nov 6. Cancer Immunol Immunother. 2023. PMID: 37932426 Free PMC article.
-
Mucosal-associated invariant T cells in hepatitis B virus-related liver failure.World J Gastroenterol. 2020 Aug 21;26(31):4703-4717. doi: 10.3748/wjg.v26.i31.4703. World J Gastroenterol. 2020. PMID: 32884227 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

