Cytome micronucleus assays with a metabolically competent human derived liver cell line (Huh6): A promising approach for routine testing of chemicals?
- PMID: 30408237
- PMCID: PMC6492180
- DOI: 10.1002/em.22254
Cytome micronucleus assays with a metabolically competent human derived liver cell line (Huh6): A promising approach for routine testing of chemicals?
Abstract
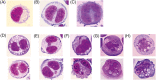
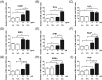
One of the main problems of in vitro genotoxicity tests is the inadequate representation of drug metabolizing enzymes in most indicator cell lines which are currently used. We identified recently a human derived liver cell line (Huh6) which detected induction of DNA damage by representatives of different groups of promutagens without enzyme mix and showed that these cells are more suitable in terms of reproducibility and sensitivity as other currently used liver derived lines. We developed a protocol for micronucleus (MN) cytome assays with these cells and validated the procedure in experiments with representatives of different groups of directly and indirectly acting genotoxic carcinogens (MMS, cisplatin, PhIP, IQ, NDMA, B(a)P, AFB1, etoposide, and H2 O2 ). The optimal cytochalasin B concentration in combination with 48 hr treatment was found to be 1.5 μg/mL and leads to a cytokinesis block proliferation index in the range between 1.7 and 2.0. The morphological characteristics of different nuclear anomalies which reflect DNA damage (MN, nuclear bridges, and buds) and their baseline frequencies in untreated cells were characterized, and the rates which are required to cause significant effects were calculated. All compounds caused dose dependent induction of MN when the cells were treated for 24 hr, longer and shorter exposure times were less effective. Experiments with different serum levels (fetal bovine serum [FBS]) showed that 10% FBS in the medium (instead of 4%) causes a substantial increase of the sensitivity of the cells. Our results indicate that the new protocol is a promising approach for routine testing of chemicals. Environ. Mol. Mutagen. 60: 134-144, 2019. © 2018 The Authors. Environmental and Molecular Mutagenesis published by Wiley Periodicals, Inc. on behalf of Environmental Mutagen Society.
Keywords: Huh6; micronuclei; nucleoplasmatic bridges; serum.
© 2018 The Authors. Environmental and Molecular Mutagenesis published by Wiley Periodicals, Inc. on behalf of Environmental Mutagen Society.
Conflict of interest statement
The authors declared that they have no conflict of interest.
Figures


Similar articles
-
Testing chemical agents with the cytokinesis-block micronucleus cytome assay.Folia Biol (Praha). 2012;58(5):215-20. Folia Biol (Praha). 2012. PMID: 23249641
-
Micronucleus assays with the human derived liver cell line (Huh6): A promising approach to reduce the use of laboratory animals in genetic toxicology.Food Chem Toxicol. 2021 Aug;154:112355. doi: 10.1016/j.fct.2021.112355. Epub 2021 Jun 17. Food Chem Toxicol. 2021. PMID: 34147571
-
Does the recommended lymphocyte cytokinesis-block micronucleus assay for human biomonitoring actually detect DNA damage induced by occupational and environmental exposure to genotoxic chemicals?Mutagenesis. 2013 Jul;28(4):375-80. doi: 10.1093/mutage/get026. Epub 2013 May 3. Mutagenesis. 2013. PMID: 23644166 Review.
-
Insensitivity of the in vitro cytokinesis-block micronucleus assay with human lymphocytes for the detection of DNA damage present at the start of the cell culture.Mutagenesis. 2012 Nov;27(6):743-7. doi: 10.1093/mutage/ges041. Epub 2012 Aug 6. Mutagenesis. 2012. PMID: 22869611
-
Use of human derived liver cells for the detection of genotoxins in comet assays.Mutat Res Genet Toxicol Environ Mutagen. 2019 Sep;845:402995. doi: 10.1016/j.mrgentox.2018.12.003. Epub 2018 Dec 10. Mutat Res Genet Toxicol Environ Mutagen. 2019. PMID: 31561885 Review.
Cited by
-
Short Assay Design for Micronucleus Detection in Human Lymphocytes.Biomed Res Int. 2021 Sep 11;2021:2322257. doi: 10.1155/2021/2322257. eCollection 2021. Biomed Res Int. 2021. PMID: 34552982 Free PMC article. Review.
-
Determination of Lymphocyte Cytokinesis-Block Micronucleus Values in Apparently Healthy Children by means of Age and Sex.Biomed Res Int. 2019 Dec 25;2019:8729561. doi: 10.1155/2019/8729561. eCollection 2019. Biomed Res Int. 2019. PMID: 31950057 Free PMC article.
-
Three-dimensional HepaRG spheroids as a liver model to study human genotoxicity in vitro with the single cell gel electrophoresis assay.Sci Rep. 2019 Jul 22;9(1):10548. doi: 10.1038/s41598-019-47114-7. Sci Rep. 2019. PMID: 31332230 Free PMC article.
-
Development of a micronucleus test using the EpiAirway™ organotypic human airway model.Genes Environ. 2023 Apr 12;45(1):14. doi: 10.1186/s41021-023-00269-2. Genes Environ. 2023. PMID: 37046355 Free PMC article.
References
-
- Doi I. 1976. Establishment of a cell line and its clonal sublines from a patient with Hepatoblastoma. Gann 67(1):1–10. - PubMed
-
- Elovaara E, Mikkola J, Stockmann‐Juvala H, Luukkanen L, Keski‐Hynnila H, Kostiainen R, Pasanen M, Pelkonen O, Vainio H. 2007. Polycyclic aromatic hydrocarbon (PAH) metabolizing enzyme activities in human lung, and their inducibility by exposure to naphthalene, phenanthrene, pyrene, chrysene, and benzo(a)pyrene as shown in the rat lung and liver. Arch Toxicol 81(3):169–182. - PubMed
-
- Fenech M. 2007. Cytokinesis‐block micronucleus cytome assay. Nat Protoc 2(5):1084–1104. - PubMed
-
- Fenech M, Kirsch‐Volders M, Rossnerova A, Sram R, Romm H, Bolognesi C, Ramakumar A, Soussaline F, Schunck C, Elhajouji A, et al. 2013. HUMN project initiative and review of validation, quality control and prospects for further development of automated micronucleus assays using image cytometry systems. Int J Hyg Environ Health 216(5):541–552. - PubMed
-
- Fowler P, Smith R, Smith K, Young J, Jeffrey L, Kirkland D, Pfuhler S, Carmichael P. 2012. Reduction of misleading ("false") positive results in mammalian cell genotoxicity assays. II. Importance of accurate toxicity measurement. Mutat Res 747(1):104–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

