Exercise training remodels human skeletal muscle mitochondrial fission and fusion machinery towards a pro-elongation phenotype
- PMID: 30408342
- PMCID: PMC6416060
- DOI: 10.1111/apha.13216
Exercise training remodels human skeletal muscle mitochondrial fission and fusion machinery towards a pro-elongation phenotype
Abstract
Aims: Mitochondria exist as a morphologically plastic network driven by cellular bioenergetic demand. Induction of fusion and fission machinery allows the organelle to regulate quality control and substrate flux. Physiological stressors promote fragmentation of the mitochondrial network, a process implicated in the onset of metabolic disease, including type 2 diabetes and obesity. It is well-known that exercise training improves skeletal muscle mitochondrial volume, number, and density. However, the effect of exercise training on muscle mitochondrial dynamics remains unclear.
Methods: Ten sedentary adults (65.8 ± 4.6 years; 34.3 ± 2.4 kg/m2 ) underwent 12 weeks of supervised aerobic exercise training (5 day/wk, 85% of HRMAX ). Body composition, cardio-metabolic testing, hyperinsulinaemic-euglycaemic clamps, and skeletal muscle biopsies were performed before and after training. MFN1, MFN2, OPA1, OMA1, FIS1, Parkin, PGC-1α, and HSC70 protein expression was assessed via Western blot.
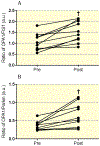
Results: Exercise training led to improvements in insulin sensitivity, aerobic capacity, and fat oxidation (all P < 0.01), as well as reductions in body weight, BMI, fat mass and fasting glucose (all P < 0.001). When normalized for changes in mitochondrial content, exercise reduced skeletal muscle FIS1 and Parkin (P < 0.05), while having no significant effect on MFN1, MFN2, OPA1, and OMA1 expression. Exercise also improved the ratio of fusion to fission proteins (P < 0.05), which positively correlated with improvements in glucose disposal (r2 = 0.59, P < 0.05).
Conclusions: Exercise training alters the expression of mitochondrial fusion and fission proteins, promoting a more fused, tubular network. These changes may contribute to the improvements in insulin sensitivity and substrate utilization that are observed after exercise training.
Keywords: exercise training; insulin resistance; mitochondrial dynamics; obesity; skeletal muscle.
© 2018 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
None of the authors have a conflict of interest related to this work.
Figures


References
-
- American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–90. - PubMed
-
- Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48(4):634–642. - PubMed
-
- Corbould A, Kim YB, Youngren JF, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288(5):E1047–1054. - PubMed
-
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

