Noninvasive Ultrasonic Drug Uncaging Maps Whole-Brain Functional Networks
- PMID: 30408444
- PMCID: PMC6274638
- DOI: 10.1016/j.neuron.2018.10.042
Noninvasive Ultrasonic Drug Uncaging Maps Whole-Brain Functional Networks
Abstract
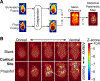
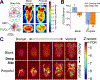
Being able to noninvasively modulate brain activity, where and when an experimenter desires, with an immediate path toward human translation is a long-standing goal for neuroscience. To enable robust perturbation of brain activity while leveraging the ability of focused ultrasound to deliver energy to any point of the brain noninvasively, we have developed biocompatible and clinically translatable nanoparticles that allow ultrasound-induced uncaging of neuromodulatory drugs. Utilizing the anesthetic propofol, together with electrophysiological and imaging assays, we show that the neuromodulatory effect of ultrasonic drug uncaging is limited spatially and temporally by the size of the ultrasound focus, the sonication timing, and the pharmacokinetics of the uncaged drug. Moreover, we see secondary effects in brain regions anatomically distinct from and functionally connected to the sonicated region, indicating that ultrasonic drug uncaging could noninvasively map the changes in functional network connectivity associated with pharmacologic action at a particular brain target.
Keywords: drug delivery; focused ultrasound; functional connectivity; functional imaging; nanotechnology; neuromodulation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Airan R (2017). Neuromodulation with nanoparticles. Science (New York, N.Y.) 357, 465. - PubMed
-
- Allan C, Kalu U-G, Sexton CE, Ebmeier KP, Kennedy S, Giacobbe P, Allan C, Herrmann L, Ebmeier K, Slotema C, et al. (2012). Transcranial stimulation in depression. The British Journal of Psychiatry : The Journal of Mental Science 200, 10–11. - PubMed
-
- Azhari H (2010). Basics of biomedical ultrasound for engineers (Wiley; ).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

