Phylogenetic, ecological and biomechanical constraints on larval form: A comparative morphological analysis of barnacle nauplii
- PMID: 30408826
- PMCID: PMC6224274
- DOI: 10.1371/journal.pone.0206973
Phylogenetic, ecological and biomechanical constraints on larval form: A comparative morphological analysis of barnacle nauplii
Abstract
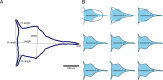
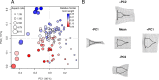
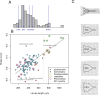
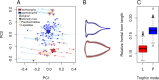
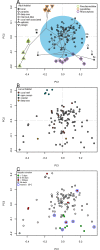
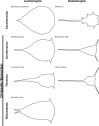
Barnacle naupliar larvae are differentiated from other zooplankton by their unique pair of frontal lateral horns, frontal filaments, and a pear-shaped cephalic shield. Their morphology impose constraints on their ecological functions and reflect their evolutionary history. To explore the potential functional basis underlying the similarities and differences in barnacle larval form, we conducted a meta-analysis on the shape of the barnacle nauplii's cephalic shield and examined its relation to larval size, trophic mode, pelagic larval duration and habitat. Nauplii cephalic shield morphology of 102 species were quantified with normalized elliptic Fourier analysis. Most of the species were distributed around the center of the morphospace but a few extreme groups occupied the periphery: nauplii that were large and lecithotrophic. Subsequent principal component regression analyses showed that larval size was a good predictor of the first shape variations axis (aspect ratio). After allometry adjustment, nauplii from different trophic modes differentiated along the second axis of the major shape variations (relative frontal horn length). Habitat was a poor predictor of variations in naupliar body form, but it could be used to differentiate extreme morphology groups from other nauplii. Our result suggests that size-related biomechanical or developmental constraints and feeding requirements are important in shaping the evolution of the naupliar body form. Within the limitations of these functional constraints, habitat drives the divergence of extreme morphology groups from the majority of species. Our comparative morphometrics analysis demonstrated how variations in larval body form can be quantitatively linked to the functional needs that constrain or drive their diversity, and inform further empirical experiments on larval functional morphology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Evolution of Feeding Shapes Swimming Kinematics of Barnacle Naupliar Larvae: A Comparison between Trophic Modes.Integr Org Biol. 2020 Apr 17;2(1):obaa011. doi: 10.1093/iob/obaa011. eCollection 2020. Integr Org Biol. 2020. PMID: 33791555 Free PMC article.
-
Swimming kinematics and hydrodynamics of barnacle larvae throughout development.Proc Biol Sci. 2020 Oct 14;287(1936):20201360. doi: 10.1098/rspb.2020.1360. Epub 2020 Oct 14. Proc Biol Sci. 2020. PMID: 33049170 Free PMC article.
-
Larval development of the pedunculate barnacles Octolasmis angulata Aurivillius 1894 and Octolasmis cor Aurivillius 1892 (Cirripedia: Thoracica: Poecilasmatidae) from the gills of the mud crab, Scylla tranquebarica Fabricius, 1798.Arthropod Struct Dev. 2015 May;44(3):253-79. doi: 10.1016/j.asd.2015.02.001. Epub 2015 Mar 10. Arthropod Struct Dev. 2015. PMID: 25770075
-
Effects of polystyrene microplastics on larval development, settlement, and metamorphosis of the intertidal barnacle Amphibalanus amphitrite.Ecotoxicol Environ Saf. 2020 May;194:110362. doi: 10.1016/j.ecoenv.2020.110362. Epub 2020 Mar 12. Ecotoxicol Environ Saf. 2020. PMID: 32171964
-
Development of the muscular and nervous systems during the larval ontogeny of the stalked barnacle, Octolasmis angulata Aurivillius 1894 (Cirripedia: Thoracicalcerea: Poecilasmatidae).Arthropod Struct Dev. 2023 Sep;76:101298. doi: 10.1016/j.asd.2023.101298. Epub 2023 Sep 4. Arthropod Struct Dev. 2023. PMID: 37672818
Cited by
-
Comparative population genetics of swimming crab host (Portunus pelagicus) and common symbiotic barnacle (Octolasmis angulata) in Vietnam.PeerJ. 2021 Jul 7;9:e11671. doi: 10.7717/peerj.11671. eCollection 2021. PeerJ. 2021. PMID: 34277149 Free PMC article.
-
Evolution of Feeding Shapes Swimming Kinematics of Barnacle Naupliar Larvae: A Comparison between Trophic Modes.Integr Org Biol. 2020 Apr 17;2(1):obaa011. doi: 10.1093/iob/obaa011. eCollection 2020. Integr Org Biol. 2020. PMID: 33791555 Free PMC article.
-
The Head of Fannia pusio (Fanniidae: Diptera) as A Novel Source of Morphometric Data for Assessing of Variation Along Geographic and Biological Lines.Zool Stud. 2021 Apr 6;60:e16. doi: 10.6620/ZS.2021.60-16. eCollection 2021. Zool Stud. 2021. PMID: 34853607 Free PMC article.
References
-
- Lai JCY, Mendoza JCE, Guinot D, Clark PF, Ng PKL. Zool Anz. 2011;250(4):407–448.
-
- Høeg JT, Møller OS. When similar beginnings lead to different ends: constraints and diversity in cirripede larval development. Invertebr Reprod Dev. 2006;49(3):125–142.
-
- Clay TW, Grünbaum D. Swimming performance as a constraint on larval morphology in plutei. Mar Ecol Prog Ser. 2011;423:185–96.
-
- Walker G. Swimming speeds of the larval stages of the parasitic barnacle, Heterosaccus lunatus (Crustacea: Cirripedia: Rhizocephala). J Mar Biol Assoc U.K. 2004;84(4):737–742. - PubMed
-
- Olesen J. The crustacean carapace—morphology, function, development, and phylogenetic history In: Watling L, Thiel M, editors. Functional morphology and diversity. New York: Oxford University Press; 2012. p. 103–139.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

