Concizumab restores thrombin generation potential in patients with haemophilia: Pharmacokinetic/pharmacodynamic modelling results of concizumab phase 1/1b data
- PMID: 30408848
- PMCID: PMC7379180
- DOI: 10.1111/hae.13627
Concizumab restores thrombin generation potential in patients with haemophilia: Pharmacokinetic/pharmacodynamic modelling results of concizumab phase 1/1b data
Abstract
Introduction: Concizumab enhances thrombin generation (TG) potential in haemophilia patients by inhibiting tissue factor pathway inhibitor (TFPI). In EXPLORER3 (phase 1b), a dose-dependent pharmacokinetic/pharmacodynamic (PK/PD) relationship was confirmed between concizumab dose, free TFPI and TG potential.
Aim: Determine the association between concizumab exposure, PD markers (free TFPI; peak TG) and bleeding episodes to establish the minimum concizumab concentration for achieving sufficient efficacy.
Methods: Free TFPI predictions were generated using an estimated concizumab-free TFPI exposure-response (Emax ) model based on concizumab phase 1/1b data for which simultaneously collected concizumab and free TFPI samples were available. Concizumab concentration at the time of a bleed was predicted using a PK model, based on available data for concizumab doses >50 μg/kg to ≤9 mg/kg. Peak TG vs concizumab concentration analyses and an Emax model were constructed based on EXPLORER3 observations.
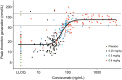
Results: The Emax model showed a tight PK/PD relationship between concizumab exposure and free TFPI; free TFPI decreased with increasing concizumab concentration. A strong correlation between concizumab concentration and peak TG was observed; concizumab >100 ng/mL re-established TG potential to within the normal reference range. Estimated EC50 values for the identified concizumab-free TFPI and concizumab-TG potential models were very similar, supporting free TFPI as an important biomarker. A correlation between bleeding episode frequency and concizumab concentration was indicated; patients with a concizumab concentration >100 ng/mL experienced less frequent bleeding. The PK model predicted that once-daily dosing would minimize within-patient concizumab PK variability.
Conclusion: Concizumab phase 2 trials will target an exposure ≥100 ng/mL, with a once-daily regimen.
Keywords: concizumab; haemophilia; modelling; pharmacodynamics; pharmacokinetics; phase 1.
© 2018 The Authors. Haemophilia Published by John Wiley & Sons Ltd.
Conflict of interest statement
HE has acted as a paid consultant for Novo Nordisk and has received funding (reimbursement for attending meetings). PA has nothing to disclose. KK has acted as an advisory board member and study investigator and has received reimbursement for attending congresses and fees for speaking from Novo Nordisk and Shire. PK has received reimbursement for attending a symposium, fees for speaking, research support and consulting fees from Novo Nordisk. JW has received grant/research/clinical trial support from Amgen, Aspen, Baxalta, Biogen Idec, Baxter Healthcare, Bayer, CSL Behring, Novo Nordisk, Octapharma, Roche, Sanofi and Shire. VJY has received reimbursement for attending symposia/congresses, and/or honoraria for speaking, and/or honoraria for consulting, and/or funds for research from Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Shire and Sobi. PHD is an employee of Novo Nordisk A/S. PC has received grants from CSL Behring, Bayer, Novo Nordisk, Pfizer and Swedish Orphan Biovitrum (AB) (publ) (Sobi), and personal fees from Bayer, Baxalta (Shire), Biogen Idec, Chugai, CSL Behring, Freeline, Novo Nordisk, Pfizer, Roche and Sobi, outside the submitted work.
Figures



Similar articles
-
A randomized trial of safety, pharmacokinetics and pharmacodynamics of concizumab in people with hemophilia A.J Thromb Haemost. 2018 Nov;16(11):2184-2195. doi: 10.1111/jth.14272. Epub 2018 Sep 30. J Thromb Haemost. 2018. PMID: 30137664 Clinical Trial.
-
Concizumab, an anti-tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay.Haemophilia. 2017 Sep;23(5):769-776. doi: 10.1111/hae.13260. Epub 2017 Jun 8. Haemophilia. 2017. PMID: 28594458
-
Inhibition of Tissue Factor Pathway Inhibitor (TFPI) as a Treatment for Haemophilia: Rationale with Focus on Concizumab.Drugs. 2018 Jun;78(9):881-890. doi: 10.1007/s40265-018-0922-6. Drugs. 2018. PMID: 29845491 Free PMC article. Review.
-
Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results.Blood. 2019 Nov 28;134(22):1973-1982. doi: 10.1182/blood.2019001542. Blood. 2019. PMID: 31444162 Free PMC article. Clinical Trial.
-
Pharmacokinetic evaluation of concizumab for the treatment of hemophilia.Expert Opin Drug Metab Toxicol. 2025 Aug;21(8):909-913. doi: 10.1080/17425255.2025.2524873. Epub 2025 Jun 25. Expert Opin Drug Metab Toxicol. 2025. PMID: 40546086 Review.
Cited by
-
Management of haemophilia A with inhibitors: A regional cross-talk.Haemophilia. 2022 Nov;28(6):950-961. doi: 10.1111/hae.14638. Epub 2022 Jul 22. Haemophilia. 2022. PMID: 35868021 Free PMC article. Review.
-
Treatment Options in Hemophilia.Dtsch Arztebl Int. 2019 Nov 22;116(47):791-798. doi: 10.3238/arztebl.2019.0791. Dtsch Arztebl Int. 2019. PMID: 31847949 Free PMC article. Review.
-
A Molecular Revolution in the Treatment of Hemophilia.Mol Ther. 2020 Apr 8;28(4):997-1015. doi: 10.1016/j.ymthe.2019.11.006. Epub 2019 Nov 13. Mol Ther. 2020. PMID: 31843450 Free PMC article. Review.
-
Current progress and future direction in the treatment for hemophilia.Int J Hematol. 2020 Jan;111(1):16-19. doi: 10.1007/s12185-019-02786-9. Epub 2019 Dec 7. Int J Hematol. 2020. PMID: 31813089 No abstract available.
-
Use of population PK/PD approach to model the thrombin generation assay: assessment in haemophilia A plasma samples spiked by a TFPI antibody.J Pharmacokinet Pharmacodyn. 2021 Aug;48(4):563-580. doi: 10.1007/s10928-021-09752-1. Epub 2021 Apr 12. J Pharmacokinet Pharmacodyn. 2021. PMID: 33846873
References
-
- Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1‐e47. - PubMed
-
- Blanchette VS, Manco‐Johnson MJ. Meeting unmet needs in inhibitor patients. Haemophilia. 2010;16(suppl 3):46‐51. - PubMed
-
- Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Hematol Am Soc Hematol Educ Prog. 2014;2014:364‐371. - PubMed
-
- Hacker MR, Geraghty S, Manco‐Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. 2001;7:392‐396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

