Exposure to mild blast forces induces neuropathological effects, neurophysiological deficits and biochemical changes
- PMID: 30409147
- PMCID: PMC6225689
- DOI: 10.1186/s13041-018-0408-1
Exposure to mild blast forces induces neuropathological effects, neurophysiological deficits and biochemical changes
Erratum in
-
Correction to: Exposure to mild blast forces induces neuropathological effects, neurophysiological deficits and biochemical changes.Mol Brain. 2021 Nov 10;14(1):164. doi: 10.1186/s13041-021-00872-w. Mol Brain. 2021. PMID: 34758856 Free PMC article. No abstract available.
Abstract
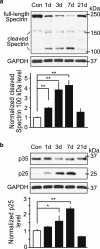
Direct or indirect exposure to an explosion can induce traumatic brain injury (TBI) of various severity levels. Primary TBI from blast exposure is commonly characterized by internal injuries, such as vascular damage, neuronal injury, and contusion, without external injuries. Current animal models of blast-induced TBI (bTBI) have helped to understand the deleterious effects of moderate to severe blast forces. However, the neurological effects of mild blast forces remain poorly characterized. Here, we investigated the effects caused by mild blast forces combining neuropathological, histological, biochemical and neurophysiological analysis. For this purpose, we employed a rodent blast TBI model with blast forces below the level that causes macroscopic neuropathological changes. We found that mild blast forces induced neuroinflammation in cerebral cortex, striatum and hippocampus. Moreover, mild blast triggered microvascular damage and axonal injury. Furthermore, mild blast caused deficits in hippocampal short-term plasticity and synaptic excitability, but no impairments in long-term potentiation. Finally, mild blast exposure induced proteolytic cleavage of spectrin and the cyclin-dependent kinase 5 activator, p35 in hippocampus. Together, these findings show that mild blast forces can cause aberrant neurological changes that critically impact neuronal functions. These results are consistent with the idea that mild blast forces may induce subclinical pathophysiological changes that may contribute to neurological and psychiatric disorders.
Keywords: Axonal swelling; Blast-induced traumatic brain injury; Calpain; Microvascular damage; Neuroinflammation; Short-term plasticity; p25.
Conflict of interest statement
Ethics approval
All animal care and experimental studies were approved by the Institutional Animal Care and Use Committees (IACUC) of West Virginia University and UT Southwestern Medical Center, and were performed according to the principles of the
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC, Cernak I, Petrie EC, Emery MJ, Swenson ER, Mayer C, Mehic E, Peskind ER, Cook DG. Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J Alzheimers Dis. 2013;37:309–323. doi: 10.3233/JAD-130182. - DOI - PMC - PubMed
-
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra160. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

