Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer's disease
- PMID: 30409172
- PMCID: PMC6225562
- DOI: 10.1186/s40478-018-0626-x
Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer's disease
Abstract
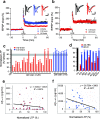
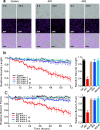
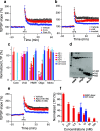
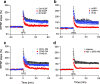
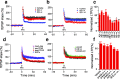
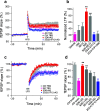
Pathologic, biochemical and genetic evidence indicates that accumulation and aggregation of amyloid β-proteins (Aβ) is a critical factor in the pathogenesis of Alzheimer's disease (AD). Several therapeutic interventions attempting to lower Aβ have failed to ameliorate cognitive decline in patients with clinical AD significantly, but most such approaches target only one or two facets of Aβ production/clearance/toxicity and do not consider the heterogeneity of human Aβ species. As synaptic dysfunction may be among the earliest deficits in AD, we used hippocampal long-term potentiation (LTP) as a sensitive indicator of the early neurotoxic effects of Aβ species. Here we confirmed prior findings that soluble Aβ oligomers, much more than fibrillar amyloid plaque cores or Aβ monomers, disrupt synaptic function. Interestingly, not all (84%) human AD brain extracts are able to inhibit LTP and the degree of LTP impairment by AD brain extracts does not correlate with Aβ levels detected by standard ELISAs. Bioactive AD brain extracts also induce neurotoxicity in iPSC-derived human neurons. Shorter forms of Aβ (including Aβ1-37, Aβ1-38, Aβ1-39), pre-Aβ APP fragments (- 30 to - 1) and N-terminally extended Aβs (- 30 to + 40) each showed much less synaptotoxicity than longer Aβs (Aβ1-42 - Aβ1-46). We found that antibodies which target the N-terminus, not the C-terminus, efficiently rescued Aβ oligomer-impaired LTP and oligomer-facilitated LTD. Our data suggest that preventing soluble Aβ oligomer formation and targeting their N-terminal residues with antibodies could be an attractive combined therapeutic approach.
Keywords: Alzheimer’s disease; Amyloid-beta protein; Long-term potentiation; Oligomers; Synaptic plasticity.
Conflict of interest statement
Competing interests
DJS is a director of and consultant to Prothena Biosciences. The other authors declare that they have no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

