Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1
- PMID: 30409173
- PMCID: PMC6225685
- DOI: 10.1186/s12974-018-1345-8
Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1
Abstract
Background: Enhancing autophagy after traumatic brain injury (TBI) may decrease the expression of neuronal apoptosis-related molecules. Autophagy-mediated neuronal survival is regulated by the sirtuin family of proteins (SIRT). Omega-3 polyunsaturated fatty acids (ω-3 PUFA) are known to have antioxidative and anti-inflammatory effects. We previously demonstrated that ω-3 PUFA supplementation attenuated neuronal apoptosis by modulating the neuroinflammatory response through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway, leading to neuroprotective effects following experimental traumatic brain injury (TBI). However, no studies have elucidated if the neuroprotective effects of ω-3 PUFAs against TBI-induced neuronal apoptosis are modulated by SIRT1-mediated deacetylation of the autophagy pathway.
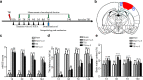
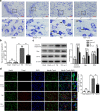
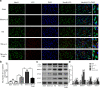
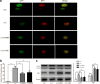
Methods: The Feeney DM TBI model was adopted to induce TBI rats. Modified neurological severity scores, the rotarod test, brain water content, and Nissl staining were employed to determine the neuroprotective effects of ω-3 PUFA supplementation. Immunofluorescent staining and western blot analysis were used to detect Beclin-1 nuclear translocation and autophagy pathway activation. The impact of SIRT1 deacetylase activity on Beclin-1 acetylation and the interaction between cytoplasmic Beclin-1 and Bcl-2 were assessed to evaluate the neuroprotective effects of ω-3 PUFAs and to determine if these effects were dependent on SIRT1-mediated deacetylation of the autophagy pathway in order to gain further insight into the mechanisms underlying the development of neuroprotection after TBI.
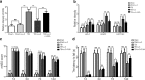
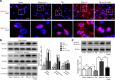
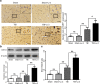
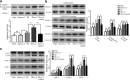
Results: ω-3 PUFA supplementation protected neurons against TBI-induced neuronal apoptosis via enhancement of the autophagy pathway. We also found that treatment with ω-3 PUFA significantly increased the NAD+/NADH ratio and SIRT1 activity following TBI. In addition, ω-3 PUFA supplementation increased Beclin-1 deacetylation and its nuclear export and induced direct interactions between cytoplasmic Beclin-1 and Bcl-2 by increasing SIRT1 activity following TBI. These events led to the inhibition of neuronal apoptosis and to neuroprotective effects through enhancing autophagy after TBI, possibly due to elevated SIRT1.
Conclusions: ω-3 PUFA supplementation attenuated TBI-induced neuronal apoptosis by inducing the autophagy pathway through the upregulation of SIRT1-mediated deacetylation of Beclin-1.
Keywords: Apoptosis; Autophagy; Omega-3 polyunsaturated fatty acid; Traumatic brain injury.
Conflict of interest statement
Ethics approval and consent to participate
The experimental protocols in the present study including all surgical procedures and animal usages conformed to the guidelines for the care and use of laboratory animals by the National Institutes of Health (NIH) and were approved by the Fujian Medical University Experimental Animal Ethics Committee (Fuzhou, China).
Consent for publication
Consent for publication is not applicable for this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Sinha SP, Avcu P, Spiegler KM, Komaravolu S, Kim K, Cominski T, Servatius RJ, Pang KC. Startle suppression after mild traumatic brain injury is associated with an increase in pro-inflammatory cytokines, reactive gliosis and neuronal loss in the caudal pontine reticular nucleus. Brain Behav Immun. 2017;61:353–364. doi: 10.1016/j.bbi.2017.01.006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

