Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction
- PMID: 30409188
- PMCID: PMC6225683
- DOI: 10.1186/s13287-018-1035-6
Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction
Abstract
Background: Autologous urothelial cells are often obtained via bladder biopsy to generate the bio-engineered urethra or bladder, while urine-derived stem cells (USC) can be obtained by a non-invasive approach. The objective of this study is to develop an optimal strategy for urothelium with permeability barrier properties using human USC which could be used for tissue repair in the urinary tract system.
Methods: USC were harvested from six healthy adult individuals. To optimize urothelial differentiation, five different differentiation methods were studied. The induced cells were assessed for gene and protein expression markers of urothelial cells via RT-PCR, Western blotting, and immunofluorescent staining. Barrier function and ultrastructure of the tight junction were assessed with permeability assays and transmission electron microscopy (TEM). Induced cells were both cultured on trans-well membranes and small intestinal submucosa, then investigated under histology analysis.
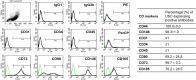
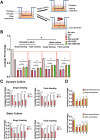
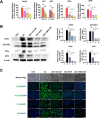
Results: Differentiated USC expressed significantly higher levels of urothelial-specific transcripts and proteins (Uroplakin III and Ia), epithelial cell markers (CK20 and AE1/AE3), and tight junction markers (ZO-1, ZO-2, E-cadherin, and Cingulin) in a time-dependent manner, compared to non-induced USC. In vitro assays using fluorescent dye demonstrated a significant reduction in permeability of differentiated USC. In addition, transmission electron microscopy confirmed appropriate ultrastructure of urothelium differentiated from USC, including tight junction formation between neighboring cells, which was similar to positive controls. Furthermore, multilayered urothelial tissues formed 2 weeks after USC were differentiated on intestine submucosal matrix.
Conclusion: The present study illustrates an optimal strategy for the generation of differentiated urothelium from stem cells isolated from the urine. The induced urothelium is phenotypically and functionally like native urothelium and has proposed uses in in vivo urological tissue repair or in vitro urethra or bladder modeling.
Keywords: Barrier function; Bladder diseases; Tight junctions; Tissue engineering; Urine-derived stem cells; Urothelium.
Conflict of interest statement
Ethics approval and consent to participate
Collection of human urine and bladder tissues in this study was approved by the Wake Forest University Health Sciences Institutional Review Board, and it was performed in accordance with the Declaration of Helsinki (2004). Signed informed consent was collected from patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Ureter tissue engineering with vessel extracellular matrix and differentiated urine-derived stem cells.Acta Biomater. 2019 Apr 1;88:266-279. doi: 10.1016/j.actbio.2019.01.072. Epub 2019 Feb 1. Acta Biomater. 2019. PMID: 30716556
-
Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering.Biomaterials. 2011 Feb;32(5):1317-26. doi: 10.1016/j.biomaterials.2010.10.006. Epub 2010 Nov 4. Biomaterials. 2011. PMID: 21055807
-
Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion.Biomaterials. 2010 Dec;31(34):8889-901. doi: 10.1016/j.biomaterials.2010.07.108. Epub 2010 Aug 25. Biomaterials. 2010. PMID: 20800278
-
Urinary Tissue Engineering: Challenges and Opportunities.Sex Med Rev. 2018 Jan;6(1):35-44. doi: 10.1016/j.sxmr.2017.08.004. Epub 2017 Oct 21. Sex Med Rev. 2018. PMID: 29066225 Review.
-
Urine as a Main Effector in Urological Tissue Engineering-A Double-Edged Sword.Cells. 2020 Feb 26;9(3):538. doi: 10.3390/cells9030538. Cells. 2020. PMID: 32110928 Free PMC article. Review.
Cited by
-
In vivo safety and biodistribution profile of Klotho-enhanced human urine-derived stem cells for clinical application.Stem Cell Res Ther. 2023 Dec 10;14(1):355. doi: 10.1186/s13287-023-03595-y. Stem Cell Res Ther. 2023. PMID: 38072946 Free PMC article.
-
Comprehensive proteomic characterization of urethral stricture disease in the Chinese population.Front Mol Biosci. 2024 Jul 26;11:1401970. doi: 10.3389/fmolb.2024.1401970. eCollection 2024. Front Mol Biosci. 2024. PMID: 39130371 Free PMC article.
-
Cells Involved in Urethral Tissue Engineering: Systematic Review.Cell Transplant. 2019 Sep-Oct;28(9-10):1106-1115. doi: 10.1177/0963689719854363. Epub 2019 Jun 25. Cell Transplant. 2019. PMID: 31237144 Free PMC article.
-
Challenges of using tissue engineering methods in the treatment of hypospadias.Front Bioeng Biotechnol. 2025 Jul 2;13:1611508. doi: 10.3389/fbioe.2025.1611508. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40671937 Free PMC article. Review.
-
Biofabrication of a Tubular Model of Human Urothelial Mucosa Using Human Wharton Jelly Mesenchymal Stromal Cells.Polymers (Basel). 2021 May 13;13(10):1568. doi: 10.3390/polym13101568. Polymers (Basel). 2021. PMID: 34068343 Free PMC article.
References
-
- Akkad T, Pelzer AE, Mitterberger M, et al. Influence of intravesical potassium on pelvic floor activity in women with recurrent urinary tract infections: comparative urodynamics might lead to enhanced detection of dysfunctional voiding. BJU Int. 2007;100(5):1071–1074. - PubMed
-
- Zhang Y, Atala A. Bladder regeneration. In: Ma PX, editor. biomaterials and regenerative medicine. Cambridge: Cambridge University Press; 2015. p. 669-79.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

