Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: a proof of concept obtained on a pig model of severe radiation burn
- PMID: 30409227
- PMCID: PMC6225585
- DOI: 10.1186/s13287-018-1051-6
Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: a proof of concept obtained on a pig model of severe radiation burn
Abstract
Background: Medical management of the severe musculocutaneous radiation syndrome involves surgical intervention with debridement of necrotic tissue. Even when skin excision is replaced by specific plastic surgery, treatment of the muscle radiation injury nonetheless remains difficult, for it involves a massive muscle defect in an unpredictable environment, subject to inflammatory waves weeks to months after irradiation, which delay healing and predispose the patient to the development of fibrous scar tissue. In this study, we investigated the long-term effect of local injections of bone marrow-derived mesenchymal stromal cells (BM-MSCs), combined with plastic surgery, to treat muscle necrosis in a large animal model.
Methods: Three months after irradiation to the rump, minipigs were treated by excision of necrotic muscle tissue, vascularized flap surgery, and four injections with or without local autologous BM-MSCs, performed weekly. The quality of the muscle wound healing was examined 1 year post-surgery.
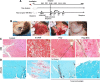
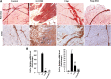
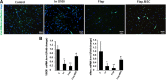
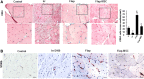
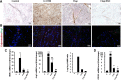
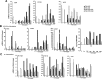
Results: The skeletal muscle surgery without MSC treatment led to permanent deposition of collagen 1 and 3, decreased myofiber diameter, failed muscle fiber regeneration, a reduced number of capillaries, and the accumulation of high calcium and fat. In animals treated by surgery and MSC injections, these indicators were substantially better and demonstrated established regeneration. MSC therapy acts at several levels by stimulating growth factors such as VEGF, which is involved in angiogenesis and satellite cell pool maintenance, and creating a macrophage M1/M2 balance.
Conclusion: Thus, cell therapy using BM-MSCs is an effective and safe way to improve recovery of irradiation-induced skeletal muscle damage without signs of long-term degeneration.
Keywords: BM-MSC; Irradiation; Muscle; Pig; Regeneration.
Conflict of interest statement
Ethics approval
All experiments procedures in this study were performed in accordance with the guidelines of the Animal Ethics Committee from French Ministry of Agriculture and approved by the Experimental Animal Ethics Committee of Jouy-en-Josas and AgroParisTech center (No.45, Avis 12-181)
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Autologous Bone Marrow Mesenchymal Stem Cells Improve the Quality and Stability of Vascularized Flap Surgery of Irradiated Skin in Pigs.Stem Cells Transl Med. 2018 Aug;7(8):569-582. doi: 10.1002/sctm.17-0267. Epub 2018 May 18. Stem Cells Transl Med. 2018. PMID: 29777577 Free PMC article.
-
Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration.Cell Tissue Res. 2018 Jun;372(3):549-570. doi: 10.1007/s00441-018-2792-3. Epub 2018 Feb 5. Cell Tissue Res. 2018. PMID: 29404727
-
Effect of Allogeneic Bone Marrow-mesenchymal Stem Cells (BM-MSCs) to Accelerate Burn Healing of Rat on the Expression of Collagen Type I and Integrin α2β1.Pak J Biol Sci. 2016;19(8-9):345-351. doi: 10.3923/pjbs.2016.345.351. Pak J Biol Sci. 2016. PMID: 29023021
-
The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration.Regen Ther. 2020 Nov 28;15:285-294. doi: 10.1016/j.reth.2020.11.002. eCollection 2020 Dec. Regen Ther. 2020. PMID: 33426231 Free PMC article. Review.
-
Mesenchymal Stromal Cell Preconditioning: The Next Step Toward a Customized Treatment For Severe Burn.Stem Cells Dev. 2018 Oct 15;27(20):1385-1405. doi: 10.1089/scd.2018.0094. Epub 2018 Jul 23. Stem Cells Dev. 2018. PMID: 30039742 Review.
Cited by
-
Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration.Front Bioeng Biotechnol. 2021 May 13;9:652970. doi: 10.3389/fbioe.2021.652970. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34095095 Free PMC article. Review.
-
Research progress on exosomes derived from mesenchymal stem cells in hematological malignancies.Hematol Oncol. 2021 Apr;39(2):162-169. doi: 10.1002/hon.2793. Epub 2020 Sep 1. Hematol Oncol. 2021. PMID: 32869900 Free PMC article. Review.
-
Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice.Stem Cell Res Ther. 2022 Jun 3;13(1):226. doi: 10.1186/s13287-022-02895-z. Stem Cell Res Ther. 2022. PMID: 35659361 Free PMC article.
-
Regenerative medicine in cardiovascular disease.Regen Ther. 2024 Oct 5;26:859-866. doi: 10.1016/j.reth.2024.09.004. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39430582 Free PMC article. Review.
-
Macrophage Identification In Situ.Biomedicines. 2021 Oct 4;9(10):1393. doi: 10.3390/biomedicines9101393. Biomedicines. 2021. PMID: 34680510 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

