Deciphering phenotypic variance in different models of DNA-PKcs deficiency
- PMID: 30409670
- PMCID: PMC6312468
- DOI: 10.1016/j.dnarep.2018.10.004
Deciphering phenotypic variance in different models of DNA-PKcs deficiency
Abstract
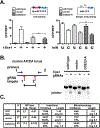
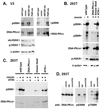
DNA-PKcs deficiency has been studied in numerous animal models and cell culture systems. In previous studies of kinase inactivating mutations in cell culture systems, ablation of DNA-PK's catalytic activity results in a cell phenotype that is virtually indistinguishable from that ascribed to complete loss of the enzyme. However, a recent compelling study demonstrates a remarkably more severe phenotype in mice harboring a targeted disruption of DNA-PK's ATP binding site as compared to DNA-PKcs deficient mice. Here we investigate the mechanism for these divergent results. We find that kinase inactivating DNA-PKcs mutants markedly radiosensitize immortalized DNA-PKcs deficient cells, but have no substantial effects on transformed DNA-PKcs deficient cells. Since the non-homologous end joining mechanism likely functions similarly in all of these cell strains, it seems unlikely that kinase inactive DNA-PK could impair the end joining mechanism in some cell types, but not in others. In fact, we observed no significant differences in either episomal or chromosomal end joining assays in cells expressing kinase inactivated DNA-PKcs versus no DNA-PKcs. Several potential explanations could explain these data including a non-catalytic role for DNA-PKcs in promoting cell death, or alteration of gene expression by loss of DNA-PKcs as opposed to inhibition of its catalytic activity. Finally, controversy exists as to whether DNA-PKcs autophosphorylates or is the target of other PIKKs; we present data demonstrating that DNA-PK primarily autophosphorylates.
Keywords: DNA dependent protein kinase; DNA-PKcs; Non-homologous end joining (NHEJ).
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Restoration of ATM Expression in DNA-PKcs-Deficient Cells Inhibits Signal End Joining.J Immunol. 2016 Apr 1;196(7):3032-42. doi: 10.4049/jimmunol.1501654. Epub 2016 Feb 26. J Immunol. 2016. PMID: 26921311 Free PMC article.
-
Telomere dysfunction and DNA-PKcs deficiency: characterization and consequence.Cancer Res. 2009 Mar 1;69(5):2100-7. doi: 10.1158/0008-5472.CAN-08-2854. Epub 2009 Feb 24. Cancer Res. 2009. PMID: 19244120 Free PMC article.
-
DNA repair kinetics in SCID mice Sertoli cells and DNA-PKcs-deficient mouse embryonic fibroblasts.Chromosoma. 2017 Mar;126(2):287-298. doi: 10.1007/s00412-016-0590-9. Epub 2016 May 2. Chromosoma. 2017. PMID: 27136939 Free PMC article.
-
DNA-PKcs deficiency in human: long predicted, finally found.Curr Opin Allergy Clin Immunol. 2009 Dec;9(6):503-9. doi: 10.1097/ACI.0b013e3283327e41. Curr Opin Allergy Clin Immunol. 2009. PMID: 19823081 Review.
-
DNA-PKcs: A promising therapeutic target in human hepatocellular carcinoma?DNA Repair (Amst). 2016 Nov;47:12-20. doi: 10.1016/j.dnarep.2016.10.004. Epub 2016 Oct 15. DNA Repair (Amst). 2016. PMID: 27789167 Review.
Cited by
-
Catalytically inactive DNA ligase IV promotes DNA repair in living cells.Nucleic Acids Res. 2022 Oct 28;50(19):11058-11071. doi: 10.1093/nar/gkac913. Nucleic Acids Res. 2022. PMID: 36263813 Free PMC article.
-
Synergistic Roles of Non-Homologous End Joining and Homologous Recombination in Repair of Ionizing Radiation-Induced DNA Double Strand Breaks in Mouse Embryonic Stem Cells.Cells. 2024 Aug 30;13(17):1462. doi: 10.3390/cells13171462. Cells. 2024. PMID: 39273031 Free PMC article.
-
DNA-PKcs phosphorylation at the T2609 cluster alters the repair pathway choice during immunoglobulin class switch recombination.Proc Natl Acad Sci U S A. 2020 Sep 15;117(37):22953-22961. doi: 10.1073/pnas.2007455117. Epub 2020 Aug 31. Proc Natl Acad Sci U S A. 2020. PMID: 32868446 Free PMC article.
-
The recent advances in non-homologous end-joining through the lens of lymphocyte development.DNA Repair (Amst). 2020 Oct;94:102874. doi: 10.1016/j.dnarep.2020.102874. Epub 2020 Jun 25. DNA Repair (Amst). 2020. PMID: 32623318 Free PMC article. Review.
-
Secrets of DNA-PKcs beyond DNA repair.NPJ Precis Oncol. 2024 Jul 23;8(1):154. doi: 10.1038/s41698-024-00655-1. NPJ Precis Oncol. 2024. PMID: 39043779 Free PMC article. Review.
References
-
- Bosma GC, Custer RP, Bosma MJ, A severe combined immunodeficiency mutation in the mouse, Nature, 301 (1983) 527–530. - PubMed
-
- Meek K, Kienker L, Dallas C, Wang W, Dark MJ, Venta PJ, Huie ML, Hirschhorn R, Bell T, SCID in Jack Russell terriers: a new animal model of DNA-PKcs deficiency, Journal of immunology, 167 (2001) 2142–2150. - PubMed
-
- Moore JC, Tang Q, Yordan NT, Moore FE, Garcia EG, Lobbardi R, Ramakrishnan A, Marvin DL, Anselmo A, Sadreyev RI, Langenau DM, Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish, The Journal of experimental medicine, 213 (2016) 2575–2589. - PMC - PubMed
-
- Bogue MA, Jhappan C, Roth DB, Analysis of variable (diversity) joining recombination in DNAdependent protein kinase (DNA-PK)-deficient mice reveals DNA-PK-independent pathways for both signal and coding joint formation, Proceedings of the National Academy of Sciences of the United States of America, 95 (1998) 15559–15564. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

