Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis
- PMID: 30409774
- PMCID: PMC6222274
- DOI: 10.1136/bmj.k4226
Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis
Abstract
Objective: To provide a complete toxicity profile, toxicity spectrum, and a safety ranking of immune checkpoint inhibitor (ICI) drugs for treatment of cancer.
Design: Systematic review and network meta-analysis.
Data sources: Electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) were systematically searched to include relevant studies published in English between January 2007 and February 2018.
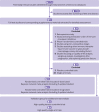
Review methods: Only head-to-head phase II and III randomised controlled trials comparing any two or three of the following treatments or different doses of the same ICI drug were included: nivolumab, pembrolizumab, ipilimumab, tremelimumab, atezolizumab, conventional therapy (chemotherapy, targeted therapy, and their combinations), two ICI drugs, or one ICI drug with conventional therapy. Eligible studies must have reported site, organ, or system level data on treatment related adverse events. High quality, single arm trials and placebo controlled trials on ICI drugs were selected to establish a validation group.
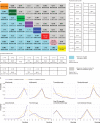
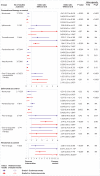
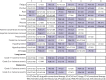
Results: 36 head-to-head phase II and III randomised trials (n=15 370) were included. The general safety of ICI drugs ranked from high to low for all adverse events was as follows: atezolizumab (probability 76%, pooled incidence 66.4%), nivolumab (56%, 71.8%), pembrolizumab (55%, 75.1%), ipilimumab (55%, 86.8%), and tremelimumab (54%, not applicable). The general safety of ICI drugs ranked from high to low for severe or life threatening adverse events was as follows: atezolizumab (49%, 15.1%), nivolumab (46%, 14.1%), pembrolizumab (72%, 19.8%), ipilimumab (51%, 28.6%), and tremelimumab (28%, not applicable). Compared with conventional therapy, treatment-related adverse events for ICI drugs occurred mainly in the skin, endocrine, hepatic, and pulmonary systems. Taking one ICI drug was generally safer than taking two ICI drugs or one ICI drug with conventional therapy. Among the five ICI drugs, atezolizumab had the highest risk of hypothyroidism, nausea, and vomiting. The predominant treatment-related adverse events for pembrolizumab were arthralgia, pneumonitis, and hepatic toxicities. The main treatment-related adverse events for ipilimumab were skin, gastrointestinal, and renal toxicities. Nivolumab had a narrow and mild toxicity spectrum, mainly causing endocrine toxicities. Integrated evidence from the pooled incidences, subgroup, and sensitivity analyses implied that nivolumab is the best option in terms of safety, especially for the treatment of lung cancer.
Conclusions: Compared with other ICI drugs used to treat cancer, atezolizumab had the best safety profile in general, and nivolumab had the best safety profile in lung cancer when taking an integrated approach. The safety ranking of treatments based on ICI drugs is modulated by specific treatment-related adverse events.
Systematic review registration: PROSPERO CRD42017082553.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Natural Science Foundation of Guang Dong Province, Health and Medical Collaborative Innovation Project of Guangzhou City, Innovation Team Development Plan of the Ministry of Education, and Overseas Expertise Introduction Project for Discipline Innovation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. version 2. 2018. https://www.nccn.org/professionals/physician_gls/default.aspx.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
