A new BCR-ABL1 Drosophila model as a powerful tool to elucidate the pathogenesis and progression of chronic myeloid leukemia
- PMID: 30409797
- PMCID: PMC6442973
- DOI: 10.3324/haematol.2018.198267
A new BCR-ABL1 Drosophila model as a powerful tool to elucidate the pathogenesis and progression of chronic myeloid leukemia
Abstract
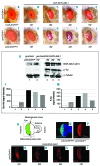
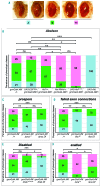
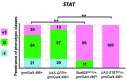
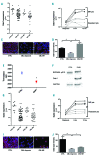
The oncoprotein BCR-ABL1 triggers chronic myeloid leukemia. It is clear that the disease relies on constitutive BCR-ABL1 kinase activity, but not all the interactors and regulators of the oncoprotein are known. We describe and validate a Drosophila leukemia model based on inducible human BCR-ABL1 expression controlled by tissue-specific promoters. The model was conceived to be a versatile tool for performing genetic screens. BCR-ABL1 expression in the developing eye interferes with ommatidia differentiation and expression in the hematopoietic precursors increases the number of circulating blood cells. We show that BCR-ABL1 interferes with the pathway of endogenous dAbl with which it shares the target protein Ena. Loss of function of ena or Dab, an upstream regulator of dAbl, respectively suppresses or enhances both the BCR-ABL1-dependent phenotypes. Importantly, in patients with leukemia decreased human Dab1 and Dab2 expression correlates with more severe disease and Dab1 expression reduces the proliferation of leukemia cells. Globally, these observations validate our Drosophila model, which promises to be an excellent system for performing unbiased genetic screens aimed at identifying new BCR-ABL1 interactors and regulators in order to better elucidate the mechanism of leukemia onset and progression.
Copyright© 2019 Ferrata Storti Foundation.
Figures


Similar articles
-
TIF1β activates leukemic transcriptional program in HSCs and promotes BCR::ABL1-induced myeloid leukemia.Leukemia. 2024 Jun;38(6):1275-1286. doi: 10.1038/s41375-024-02276-w. Epub 2024 May 11. Leukemia. 2024. PMID: 38734786
-
miR-96 acts as a tumor suppressor via targeting the BCR-ABL1 oncogene in chronic myeloid leukemia blastic transformation.Biomed Pharmacother. 2019 Nov;119:109413. doi: 10.1016/j.biopha.2019.109413. Epub 2019 Sep 10. Biomed Pharmacother. 2019. PMID: 31518872
-
BCR-ABL1 mediated miR-150 downregulation through MYC contributed to myeloid differentiation block and drug resistance in chronic myeloid leukemia.Haematologica. 2018 Dec;103(12):2016-2025. doi: 10.3324/haematol.2018.193086. Epub 2018 Jul 26. Haematologica. 2018. PMID: 30049824 Free PMC article.
-
CML - Not only BCR-ABL1 matters.Best Pract Res Clin Haematol. 2020 Sep;33(3):101194. doi: 10.1016/j.beha.2020.101194. Epub 2020 Jun 7. Best Pract Res Clin Haematol. 2020. PMID: 33038988 Review.
-
Early BCR-ABL1 Reduction Is Predictive of Better Event-free Survival in Patients With Newly Diagnosed Chronic Myeloid Leukemia Treated With Any Tyrosine Kinase Inhibitor.Clin Lymphoma Myeloma Leuk. 2016 Aug;16 Suppl:S96-S100. doi: 10.1016/j.clml.2016.03.008. Epub 2016 Apr 1. Clin Lymphoma Myeloma Leuk. 2016. PMID: 27131622 Review.
Cited by
-
Development of BCR-ABL1 Transgenic Zebrafish Model Reproducing Chronic Myeloid Leukemia (CML) Like-Disease and Providing a New Insight into CML Mechanisms.Cells. 2021 Feb 19;10(2):445. doi: 10.3390/cells10020445. Cells. 2021. PMID: 33669758 Free PMC article.
-
Expression of chronic myeloid leukemia oncogenes BCR-ABL P210 and BCR-ABL T315I affect cellular and humoral innate immunity in Drosophila melanogaster.MicroPubl Biol. 2022 Apr 13;2022:10.17912/micropub.biology.000551. doi: 10.17912/micropub.biology.000551. eCollection 2022. MicroPubl Biol. 2022. PMID: 35622506 Free PMC article.
-
Identifying the personalized driver gene sets maximally contributing to abnormality of transcriptome phenotype in glioblastoma multiforme individuals.Mol Oncol. 2023 Nov;17(11):2472-2490. doi: 10.1002/1878-0261.13499. Epub 2023 Aug 8. Mol Oncol. 2023. PMID: 37491836 Free PMC article.
-
Validation of a Drosophila model of wild-type and T315I mutated BCR-ABL1 in chronic myeloid leukemia: an effective platform for treatment screening.Haematologica. 2020 Jan 31;105(2):387-397. doi: 10.3324/haematol.2019.219394. Print 2020. Haematologica. 2020. PMID: 31101753 Free PMC article.
-
The Leukemic Fly: Promises and Challenges.Cells. 2020 Jul 21;9(7):1737. doi: 10.3390/cells9071737. Cells. 2020. PMID: 32708107 Free PMC article. Review.
References
-
- Chereda B, Melo JV. Natural course and biology of CML. Ann Hematol. 2015;94(Suppl 2):S107–121. - PubMed
-
- Gonzalez C. Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat Rev Cancer. 2013;13(3):172–183. - PubMed
-
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101(7):703–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

