Establishment of a murine culture system for modeling the temporal progression of cranial and trunk neural crest cell differentiation
- PMID: 30409814
- PMCID: PMC6307900
- DOI: 10.1242/dmm.035097
Establishment of a murine culture system for modeling the temporal progression of cranial and trunk neural crest cell differentiation
Abstract
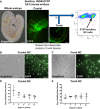
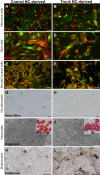
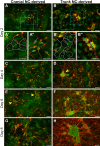
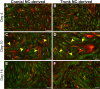
The neural crest (NC) is a transient population of embryonic progenitors that are implicated in a diverse range of congenital birth defects and pediatric syndromes. The broad spectrum of NC-related disorders can be attributed to the wide variety of differentiated cell types arising from the NC. In vitro models of NC development provide a powerful platform for testing the relative contributions of intrinsic and extrinsic factors mediating NC differentiation under normal and pathogenic conditions. Although differentiation is a dynamic process that unfolds over time, currently, there is no well-defined chronology that characterizes the in vitro progression of NC differentiation towards specific cell fates. In this study, we have optimized culture conditions for expansion of primary murine NC cells that give rise to both ectodermal and mesoectodermal derivatives, even after multiple passages. Significantly, we have delineated highly reproducible timelines that include distinct intermediate stages for lineage-specific NC differentiation in vitro In addition, isolating both cranial and trunk NC cells from the same embryos enabled us to make direct comparisons between the two cell populations over the course of differentiation. Our results define characteristic changes in cell morphology and behavior that track the temporal progression of NC cells as they differentiate along the neuronal, glial and chondrogenic lineages in vitro These benchmarks constitute a chronological baseline for assessing how genetic or environmental disruptions may facilitate or impede NC differentiation. Introducing a temporal dimension substantially increases the power of this platform for screening drugs or chemicals for developmental toxicity or therapeutic potential. This article has an associated First Person interview with the first author of the paper.
Keywords: Chondrogenic differentiation; Differentiation timeline; Glial differentiation; In vitro model of neural crest differentiation; Neuronal differentiation; Sox9.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsM.R.R., V.S.S., M.D.L., K.R.S. and A.J.U. have no conflicts of interest to disclose. V.M.L. is currently an employee of STEMCELL Technologies, which provided the Complete MesenCult™ Adipogenic Medium used in this study.
Figures



References
-
- Akamatsu W., Okano H. J., Osumi N., Inoue T., Nakamura S., Sakakibara S.-I., Miura M., Matsuo N., Darnell R. B. and Okano H. (1999). Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc. Natl. Acad. Sci. USA 96, 9885-9890. 10.1073/pnas.96.17.9885 - DOI - PMC - PubMed
-
- Akiyama H., Kim J.-E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T. et al. (2005). Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 102, 14665-14670. 10.1073/pnas.0504750102 - DOI - PMC - PubMed
-
- Anderson K. D., Morin M. A., Beckel-Mitchener A., Mobarak C. D., Neve R. L., Furneaux H. M., Burry R. and Perrone-Bizzozero N. I. (2000). Overexpression of HuD, but not of its truncated form HuD I+II, promotes GAP-43 gene expression and neurite outgrowth in PC12 cells in the absence of nerve growth factor. J. Neurochem. 75, 1103-1114. 10.1046/j.1471-4159.2000.0751103.x - DOI - PubMed
-
- Anderson K. D., Sengupta J., Morin M., Neve R. L., Valenzuela C. F. and Perrone-Bizzozero N. I. (2001). Overexpression of HuD accelerates neurite outgrowth and increases GAP-43 mRNA expression in cortical neurons and retinoic acid-induced embryonic stem cells in vitro. Exp. Neurol. 168, 250-258. 10.1006/exnr.2000.7599 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

