Digital technologies and adherence in respiratory diseases: the road ahead
- PMID: 30409819
- PMCID: PMC6364097
- DOI: 10.1183/13993003.01147-2018
Digital technologies and adherence in respiratory diseases: the road ahead
Abstract
Outcomes for patients with chronic respiratory diseases remain poor despite the development of novel therapies. In part, this reflects the fact that adherence to therapy is low and clinicians lack accurate methods to assess this issue. Digital technologies hold promise to overcome these barriers to care. For example, algorithmic analysis of large amounts of information collected on health status and treatment use, along with other disease relevant information such as environmental data, can be used to help guide personalised interventions that may have a positive health impact, such as establishing habitual and correct inhaler use. Novel approaches to data analysis also offer the possibility of statistical algorithms that are better able to predict exacerbations, thereby creating opportunities for preventive interventions that may adapt therapy as disease activity changes. To realise these possibilities, digital approaches to disease management should be supported by strong evidence, have a solid infrastructure, be designed collaboratively as clinically effective and cost-effective systems, and reflect the needs of patients and healthcare providers. Regulatory standards for digital interventions and strategies to handle the large amounts of data generated are also needed. This review highlights the opportunities provided by digital technologies for managing patients with respiratory diseases.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: J.D. Blakey reports personal fees (for an advisory board meeting) from Teva, personal fees (for travel and lectures) and non-financial support from Napp, personal fees (for a presentation) from Novartis, personal fees and non-financial support (for travel and lectures) from AstraZeneca, and personal fees and non-financial support (for travel and lectures) from Boehringer Ingelheim, outside the submitted work. B.G. Bender has nothing to disclose. Conflict of interest: A.L. Dima reports grants and non-financial support (for travel and research) from Respiratory Effectiveness Group, outside the submitted work. Conflict of interest: J. Weinman reports personal fees from Atlantis Healthcare, Boehringer Ingelheim, Chugai/Roche, Ferring, Sanofi and Teva, and grants from Merck, outside the submitted work. Conflict of interest: G. Safioti is an employee of Teva. Conflict of interest: R.W. Costello has received funding for research from Aerogen and GSK, speaker's and consultancy fees from Aerogen, Boehringer Inghelheim, AstraZeneca, GSK, Novartis and Teva. He has licensed an acoustic device to assess adherence to Vitalograph and has a patent pending for the identification of inhaler use (P10961USPC) and two pending for methods to assess adherence and remotely predict exacerbations.
Figures
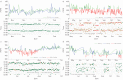
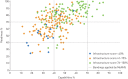
References
-
- Chan AH, Reddel HK, Apter A, et al. . Adherence monitoring and e-health: how clinicians and researchers can use technology to promote inhaler adherence for asthma. J Allergy Clin Immunol Pract 2013; 1: 446–454. - PubMed
-
- van Boven JF, Ryan D, Eakin MN, et al. . Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract 2016; 4: 835–846. - PubMed
-
- Vrijens B, Dima AL, Van Ganse E, et al. . What we mean when we talk about adherence in respiratory medicine. J Allergy Clin Immunol Pract 2016; 4: 802–812. - PubMed
-
- Chan AH, Stewart AW, Harrison J, et al. . The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med 2015; 3: 210–219. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
