IND-Enabling Studies for a Clinical Trial to Genetically Program a Persistent Cancer-Targeted Immune System
- PMID: 30409823
- PMCID: PMC6359988
- DOI: 10.1158/1078-0432.CCR-18-0963
IND-Enabling Studies for a Clinical Trial to Genetically Program a Persistent Cancer-Targeted Immune System
Abstract
Purpose: To improve persistence of adoptively transferred T-cell receptor (TCR)-engineered T cells and durable clinical responses, we designed a clinical trial to transplant genetically-modified hematopoietic stem cells (HSCs) together with adoptive cell transfer of T cells both engineered to express an NY-ESO-1 TCR. Here, we report the preclinical studies performed to enable an investigational new drug (IND) application.
Experimental design: HSCs transduced with a lentiviral vector expressing NY-ESO-1 TCR and the PET reporter/suicide gene HSV1-sr39TK and T cells transduced with a retroviral vector expressing NY-ESO-1 TCR were coadministered to myelodepleted HLA-A2/Kb mice within a formal Good Laboratory Practice (GLP)-compliant study to demonstrate safety, persistence, and HSC differentiation into all blood lineages. Non-GLP experiments included assessment of transgene immunogenicity and in vitro viral insertion safety studies. Furthermore, Good Manufacturing Practice (GMP)-compliant cell production qualification runs were performed to establish the manufacturing protocols for clinical use.
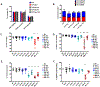
Results: TCR genetically modified and ex vivo-cultured HSCs differentiated into all blood subsets in vivo after HSC transplantation, and coadministration of TCR-transduced T cells did not result in increased toxicity. The expression of NY-ESO-1 TCR and sr39TK transgenes did not have a detrimental effect on gene-modified HSC's differentiation to all blood cell lineages. There was no evidence of genotoxicity induced by the lentiviral vector. GMP batches of clinical-grade transgenic cells produced during qualification runs had adequate stability and functionality.
Conclusions: Coadministration of HSCs and T cells expressing an NY-ESO-1 TCR is safe in preclinical models. The results presented in this article led to the FDA approval of IND 17471.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors have no conflict of interests on this work
Figures
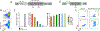

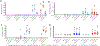


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

