EPFL Signals in the Boundary Region of the SAM Restrict Its Size and Promote Leaf Initiation
- PMID: 30409857
- PMCID: PMC6324244
- DOI: 10.1104/pp.18.00714
EPFL Signals in the Boundary Region of the SAM Restrict Its Size and Promote Leaf Initiation
Abstract
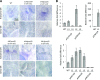
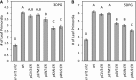
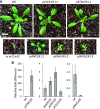
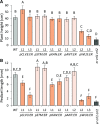
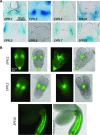
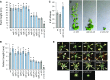
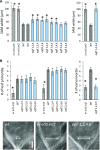
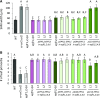
The shoot apical meristem (SAM) enables the formation of new organs throughout the life of a plant. ERECTA family (ERf) receptors restrict SAM size and promote initiation of leaves while simultaneously supporting establishment of correct phyllotaxy. In the epidermis and during organ elongation ERf activity is regulated by a family of Epidermal Patterning Factor-Like (EPFL) secreted Cys-rich small proteins. Here we show that ERfs play a critical role in communication between the SAM leaf boundary and the central zone in Arabidopsis (Arabidopsis thaliana). Ectopic expression of ERECTA in the central zone using the CLAVATA3 promoter is sufficient to restrict meristem size and promote leaf initiation. Genetic analysis demonstrated that four putative ligands: EPFL1, EPFL2, EPFL4, and EPFL6 function redundantly in the SAM. These genes are expressed at the SAM-leaf boundary and in the peripheral zone. Previously EPFL4 and EPFL6 have been linked with elongation of aboveground organs. Here we demonstrate that EPFL1 and EPFL2 promote organ elongation as well. In addition, we show that expression of ERECTA in the central zone of the SAM has a strong impact on elongation of internodes and pedicels and growth of leaves. These results suggest that ERfs can stimulate organ growth cell nonautonomously.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures

Similar articles
-
An updated model of shoot apical meristem regulation by ERECTA family and CLAVATA3 signaling pathways in Arabidopsis.Development. 2024 Jun 15;151(12):dev202870. doi: 10.1242/dev.202870. Epub 2024 Jun 24. Development. 2024. PMID: 38814747 Free PMC article.
-
ERECTA family signaling constrains CLAVATA3 and WUSCHEL to the center of the shoot apical meristem.Development. 2021 Mar 9;148(5):dev189753. doi: 10.1242/dev.189753. Development. 2021. PMID: 33593817
-
ERECTA family genes regulate auxin transport in the shoot apical meristem and forming leaf primordia.Plant Physiol. 2013 Aug;162(4):1978-91. doi: 10.1104/pp.113.218198. Epub 2013 Jul 2. Plant Physiol. 2013. PMID: 23821653 Free PMC article.
-
Diverse roles of ERECTA family genes in plant development.J Integr Plant Biol. 2013 Dec;55(12):1238-50. doi: 10.1111/jipb.12108. Epub 2013 Oct 30. J Integr Plant Biol. 2013. PMID: 24016315 Review.
-
Molecular and Hormonal Regulation of Leaf Morphogenesis in Arabidopsis.Int J Mol Sci. 2020 Jul 20;21(14):5132. doi: 10.3390/ijms21145132. Int J Mol Sci. 2020. PMID: 32698541 Free PMC article. Review.
Cited by
-
Initiation of aboveground organ primordia depends on combined action of auxin, ERECTA family genes, and PINOID.Plant Physiol. 2022 Aug 29;190(1):794-812. doi: 10.1093/plphys/kiac288. Plant Physiol. 2022. PMID: 35703946 Free PMC article.
-
Comprehensive identification of cotton EPF/EPFL receptors and functional characterization of the GhEPFL1-1-GhER1 module in drought tolerance.BMC Plant Biol. 2025 Jul 11;25(1):901. doi: 10.1186/s12870-025-06797-z. BMC Plant Biol. 2025. PMID: 40646466 Free PMC article.
-
Mathematical modeling of plant cell fate transitions controlled by hormonal signals.PLoS Comput Biol. 2020 Jul 20;16(7):e1007523. doi: 10.1371/journal.pcbi.1007523. eCollection 2020 Jul. PLoS Comput Biol. 2020. PMID: 32687508 Free PMC article.
-
Small Peptides: Orchestrators of Plant Growth and Developmental Processes.Int J Mol Sci. 2024 Jul 11;25(14):7627. doi: 10.3390/ijms25147627. Int J Mol Sci. 2024. PMID: 39062870 Free PMC article. Review.
-
Advancements in Rice Leaf Development Research.Plants (Basel). 2024 Mar 21;13(6):904. doi: 10.3390/plants13060904. Plants (Basel). 2024. PMID: 38592944 Free PMC article. Review.
References
-
- Abrash EB, Bergmann DC (2010) Regional specification of stomatal production by the putative ligand CHALLAH. Development 137: 447–455 - PubMed
-
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131: 3615–3626 - PubMed
-
- Bergmann DC, Lukowitz W, Somerville CR (2004) Stomatal development and pattern controlled by a MAPKK kinase. Science 304: 1494–1497 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

