Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles
- PMID: 30409859
- PMCID: PMC6376486
- DOI: 10.1126/science.aao0666
Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles
Abstract
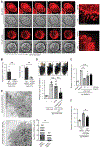
Anaphylactic reactions are triggered when allergens enter the blood circulation and activate immunoglobulin E (IgE)-sensitized mast cells (MCs), causing systemic discharge of prestored proinflammatory mediators. As MCs are extravascular, how they perceive circulating allergens remains a conundrum. Here, we describe the existence of a CD301b+ perivascular dendritic cell (DC) subset that continuously samples blood and relays antigens to neighboring MCs, which vigorously degranulate and trigger anaphylaxis. DC antigen transfer involves the active discharge of surface-associated antigens on 0.5- to 1.0-micrometer microvesicles (MVs) generated by vacuolar protein sorting 4 (VPS4). Antigen sharing by DCs is not limited to MCs, as neighboring DCs also acquire antigen-bearing MVs. This capacity of DCs to distribute antigen-bearing MVs to various immune cells in the perivascular space potentiates inflammatory and immune responses to blood-borne antigens.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



Comment in
-
Dangerous liaisons in anaphylaxis.Science. 2018 Nov 9;362(6415):640-641. doi: 10.1126/science.aav4505. Science. 2018. PMID: 30409871 No abstract available.
-
A Shocking Type of Communication.Immunity. 2018 Dec 18;49(6):999-1001. doi: 10.1016/j.immuni.2018.12.004. Immunity. 2018. PMID: 30566890
References
-
- Tejedor-Alonso MA, Moro-Moro M, Mugica-Garcia MV, Epidemiology of Anaphylaxis: Contributions From the Last 10 Years. J Investig Allergol Clin Immunol 25, 163–175; quiz follow 174–165 (2015). - PubMed
-
- Simons FE, O. World Allergy, World Allergy Organization survey on global availability of essentials for the assessment and management of anaphylaxis by allergy-immunology specialists in health care settings. Ann Allergy Asthma Immunol 104, 405–412 (2010). - PubMed
-
- Sampson HA et al. , Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol 115, 584–591 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

