Functional consequences of the CAPOS mutation E818K of Na+,K+-ATPase
- PMID: 30409907
- PMCID: PMC6322875
- DOI: 10.1074/jbc.RA118.004591
Functional consequences of the CAPOS mutation E818K of Na+,K+-ATPase
Abstract
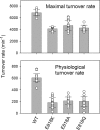
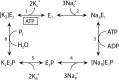
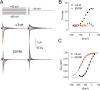
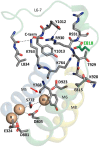
The cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) syndrome is caused by the single mutation E818K of the α3-isoform of Na+,K+-ATPase. Here, using biochemical and electrophysiological approaches, we examined the functional characteristics of E818K, as well as of E818Q and E818A mutants. We found that these amino acid substitutions reduce the apparent Na+ affinity at the cytoplasmic-facing sites of the pump protein and that this effect is more pronounced for the lysine and glutamine substitutions (3-4-fold) than for the alanine substitution. The electrophysiological measurements indicated a more conspicuous, ∼30-fold reduction of apparent Na+ affinity for the extracellular-facing sites in the CAPOS mutant, which was related to an accelerated transition between the phosphoenzyme intermediates E1P and E2P. The apparent affinity for K+ activation of the ATPase activity was unaffected by these substitutions, suggesting that primarily the Na+-specific site III is affected. Furthermore, the apparent affinities for ATP and vanadate were WT-like in E818K, indicating a normal E1-E2 equilibrium of the dephosphoenzyme. Proton-leak currents were not increased in E818K. However, the CAPOS mutation caused a weaker voltage dependence of the pumping rate and a stronger inhibition by cytoplasmic K+ than the WT enzyme, which together with the reduced Na+ affinity of the cytoplasmic-facing sites precluded proper pump activation under physiological conditions. The functional deficiencies could be traced to the participation of Glu-818 in an intricate hydrogen-bonding/salt-bridge network, connecting it to key residues involved in Na+ interaction at site III.
Keywords: ATP1A3; Na+ site; Na+,K+-pump; Na+/K+-ATPase; P-type ATPase; electrophysiology; enzyme mechanism; membrane enzyme; membrane transport; mutant; neurological disease; potassium transport; site-directed mutagenesis; sodium transport.
© 2019 Roenn et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
-
- Blanco G., and Mercer R. W. (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–F650 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
LinkOut - more resources
Full Text Sources

