Chaperome heterogeneity and its implications for cancer study and treatment
- PMID: 30409908
- PMCID: PMC6369301
- DOI: 10.1074/jbc.REV118.002811
Chaperome heterogeneity and its implications for cancer study and treatment
Abstract
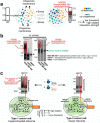
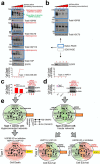
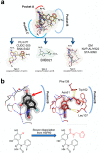
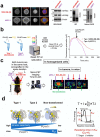
The chaperome is the collection of proteins in the cell that carry out molecular chaperoning functions. Changes in the interaction strength between chaperome proteins lead to an assembly that is functionally and structurally distinct from each constituent member. In this review, we discuss the epichaperome, the cellular network that forms when the chaperome components of distinct chaperome machineries come together as stable, functionally integrated, multimeric complexes. In tumors, maintenance of the epichaperome network is vital for tumor survival, rendering them vulnerable to therapeutic interventions that target critical epichaperome network components. We discuss how the epichaperome empowers an approach for precision medicine cancer trials where a new target, biomarker, and relevant drug candidates can be correlated and integrated. We introduce chemical biology methods to investigate the heterogeneity of the chaperome in a given cellular context. Lastly, we discuss how ligand-protein binding kinetics are more appropriate than equilibrium binding parameters to characterize and unravel chaperome targeting in cancer and to gauge the selectivity of ligands for specific tumor-associated chaperome pools.
Keywords: 70 kilodalton heat shock protein (Hsp70); biomarker; cancer biology; cancer therapy; chaperome; chaperone; chemical biology; chemical probes; epichaperome; heat shock protein 90 (Hsp90); protein networks; stress response.
© 2019 Wang et al.
Conflict of interest statement
G. C. has partial ownership in Samus Therapeutics Inc., which develops epichaperome inhibitors
Figures


References
-
- Wang X., Venable J., LaPointe P., Hutt D. M., Koulov A. V., Coppinger J., Gurkan C., Kellner W., Matteson J., Plutner H., Riordan J. R., Kelly J. W., Yates J. R. 3rd., and Balch W. E. (2006) Hsp90 co-chaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell 127, 803–815 10.1016/j.cell.2006.09.043 - DOI - PubMed
-
- Brehme M., Voisine C., Rolland T., Wachi S., Soper J. H., Zhu Y., Orton K., Villella A., Garza D., Vidal M., Ge H., and Morimoto R. I. (2014) A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 9, 1135–1150 10.1016/j.celrep.2014.09.042 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

