Dynamic acetylation of the kinetochore-associated protein HEC1 ensures accurate microtubule-kinetochore attachment
- PMID: 30409912
- PMCID: PMC6333894
- DOI: 10.1074/jbc.RA118.003844
Dynamic acetylation of the kinetochore-associated protein HEC1 ensures accurate microtubule-kinetochore attachment
Abstract
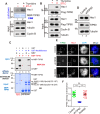
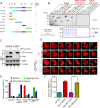
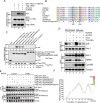
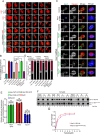
Faithful chromosome segregation during mitosis is critical for maintaining genome integrity in cell progeny and relies on accurate and robust kinetochore-microtubule attachments. The NDC80 complex, a tetramer comprising kinetochore protein HEC1 (HEC1), NDC80 kinetochore complex component NUF2 (NUF2), NDC80 kinetochore complex component SPC24 (SPC24), and SPC25, plays a critical role in kinetochore-microtubule attachment. Mounting evidence indicates that phosphorylation of HEC1 is important for regulating the binding of the NDC80 complex to microtubules. However, it remains unclear whether other post-translational modifications, such as acetylation, regulate NDC80-microtubule attachment during mitosis. Here, using pulldown assays with HeLa cell lysates and site-directed mutagenesis, we show that HEC1 is a bona fide substrate of the lysine acetyltransferase Tat-interacting protein, 60 kDa (TIP60) and that TIP60-mediated acetylation of HEC1 is essential for accurate chromosome segregation in mitosis. We demonstrate that TIP60 regulates the dynamic interactions between NDC80 and spindle microtubules during mitosis and observed that TIP60 acetylates HEC1 at two evolutionarily conserved residues, Lys-53 and Lys-59. Importantly, this acetylation weakened the phosphorylation of the N-terminal HEC1(1-80) region at Ser-55 and Ser-62, which is governed by Aurora B and regulates NDC80-microtubule dynamics, indicating functional cross-talk between these two post-translation modifications of HEC1. Moreover, the TIP60-mediated acetylation was specifically reversed by sirtuin 1 (SIRT1). Taken together, our results define a conserved signaling hierarchy, involving HEC1, TIP60, Aurora B, and SIRT1, that integrates dynamic HEC1 acetylation and phosphorylation for accurate kinetochore-microtubule attachment in the maintenance of genomic stability during mitosis.
Keywords: HEC1; NDC80 complex; NUF2; TIP60; chromosomal segregation; kinetochore; microtubule; mitosis; mitotic spindle; protein acylation; spindle assembly.
© 2019 Zhao et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
The Hec1/Ndc80 tail domain is required for force generation at kinetochores, but is dispensable for kinetochore-microtubule attachment formation and Ska complex recruitment.Mol Biol Cell. 2020 Jul 1;31(14):1453-1473. doi: 10.1091/mbc.E20-05-0286. Epub 2020 May 13. Mol Biol Cell. 2020. PMID: 32401635 Free PMC article.
-
The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis.Mol Biol Cell. 2011 Mar 15;22(6):759-68. doi: 10.1091/mbc.E10-08-0671. Epub 2011 Jan 26. Mol Biol Cell. 2011. PMID: 21270439 Free PMC article.
-
Chmp4c is required for stable kinetochore-microtubule attachments.Chromosoma. 2018 Dec;127(4):461-473. doi: 10.1007/s00412-018-0675-8. Epub 2018 Jul 2. Chromosoma. 2018. PMID: 29968190
-
Hec1/Ndc80 Tail Domain Function at the Kinetochore-Microtubule Interface.Front Cell Dev Biol. 2020 Feb 26;8:43. doi: 10.3389/fcell.2020.00043. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32161753 Free PMC article. Review.
-
Kinetochore-microtubule coupling mechanisms mediated by the Ska1 complex and Cdt1.Essays Biochem. 2020 Sep 4;64(2):337-347. doi: 10.1042/EBC20190075. Essays Biochem. 2020. PMID: 32844209 Free PMC article. Review.
Cited by
-
The TOG protein Stu2 is regulated by acetylation.PLoS Genet. 2022 Sep 9;18(9):e1010358. doi: 10.1371/journal.pgen.1010358. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36084134 Free PMC article.
-
Recent Progress on the Localization of the Spindle Assembly Checkpoint Machinery to Kinetochores.Cells. 2019 Mar 23;8(3):278. doi: 10.3390/cells8030278. Cells. 2019. PMID: 30909555 Free PMC article. Review.
-
Phosphorylation of Bub1 by Mph1 promotes Bub1 signaling at the kinetochore to ensure accurate chromosome segregation.J Biol Chem. 2024 Jan;300(1):105559. doi: 10.1016/j.jbc.2023.105559. Epub 2023 Dec 13. J Biol Chem. 2024. PMID: 38097187 Free PMC article.
-
TIP60 acetylation of Bub1 regulates centromeric H2AT120 phosphorylation for faithful chromosome segregation.Sci China Life Sci. 2024 Sep;67(9):1957-1969. doi: 10.1007/s11427-023-2604-8. Epub 2024 May 17. Sci China Life Sci. 2024. PMID: 38763998
-
Fluorescence complementation-based FRET imaging reveals centromere assembly dynamics.Mol Biol Cell. 2024 Apr 1;35(4):ar51. doi: 10.1091/mbc.E23-09-0379. Epub 2024 Feb 21. Mol Biol Cell. 2024. PMID: 38381564 Free PMC article.
References
-
- McCleland M. L., Kallio M. J., Barrett-Wilt G. A., Kestner C. A., Shabanowitz J., Hunt D. F., Gorbsky G. J., and Stukenberg P. T. (2004) The vertebrate NDC80 complex contains SPC24 and SPC25 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr. Biol. 14, 131–137 10.1016/j.cub.2003.12.058 - DOI - PubMed
-
- McCleland M. L., Gardner R. D., Kallio M. J., Daum J. R., Gorbsky G. J., Burke D. J., and Stukenberg P. T. (2003) The highly conserved NDC80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101–114 10.1101/gad.1040903 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

