Dietary metabolism, the gut microbiome, and heart failure
- PMID: 30410105
- PMCID: PMC6377322
- DOI: 10.1038/s41569-018-0108-7
Dietary metabolism, the gut microbiome, and heart failure
Abstract
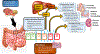
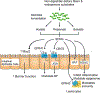
Advances in our understanding of how the gut microbiota contributes to human health and diseases have expanded our insight into how microbial composition and function affect the human host. Heart failure is associated with splanchnic circulation congestion, leading to bowel wall oedema and impaired intestinal barrier function. This situation is thought to heighten the overall inflammatory state via increased bacterial translocation and the presence of bacterial products in the systemic blood circulation. Several metabolites produced by gut microorganisms from dietary metabolism have been linked to pathologies such as atherosclerosis, hypertension, heart failure, chronic kidney disease, obesity, and type 2 diabetes mellitus. These findings suggest that the gut microbiome functions like an endocrine organ by generating bioactive metabolites that can directly or indirectly affect host physiology. In this Review, we discuss several newly discovered gut microbial metabolic pathways, including the production of trimethylamine and trimethylamine N-oxide, short-chain fatty acids, and secondary bile acids, that seem to participate in the development and progression of cardiovascular diseases, including heart failure. We also discuss the gut microbiome as a novel therapeutic target for the treatment of cardiovascular disease, and potential strategies for targeting intestinal microbial processes.
Figures



References
-
- Yancy CW et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, 1810–1852, doi: 10.1161/CIR.0b013e31829e8807 (2013). - DOI - PubMed
-
- Yancy CW et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 70, 776–803, doi: 10.1016/j.jacc.2017.04.025 (2017). - DOI - PubMed
-
- Anker SD et al. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol 79, 1426–1430 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

