Imaging Biomarkers for the Diagnosis and Prognosis of Neurodegenerative Diseases. The Example of Amyotrophic Lateral Sclerosis
- PMID: 30410433
- PMCID: PMC6209630
- DOI: 10.3389/fnins.2018.00784
Imaging Biomarkers for the Diagnosis and Prognosis of Neurodegenerative Diseases. The Example of Amyotrophic Lateral Sclerosis
Abstract
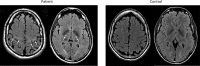
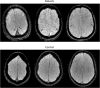
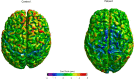
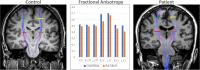
The term amyotrophic lateral sclerosis (ALS) comprises a heterogeneous group of fatal neurodegenerative disorders of largely unknown etiology characterized by the upper motor neurons (UMN) and/or lower motor neurons (LMN) degeneration. The development of brain imaging biomarkers is essential to advance in the diagnosis, stratification and monitoring of ALS, both in the clinical practice and clinical trials. In this review, the characteristics of an optimal imaging biomarker and common pitfalls in biomarkers evaluation will be discussed. Moreover, the development and application of the most promising brain magnetic resonance (MR) imaging biomarkers will be reviewed. Finally, the integration of both qualitative and quantitative multimodal brain MR biomarkers in a structured report will be proposed as a support tool for ALS diagnosis and stratification.
Keywords: a structured report; amyotrophic lateral sclerosis; brain MR image analysis; imaging biomarkers; neurodegenerative disorders.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

