A Natural Variant of the Signaling Molecule Vav1 Enhances Susceptibility to Myasthenia Gravis and Influences the T Cell Receptor Repertoire
- PMID: 30410484
- PMCID: PMC6210741
- DOI: 10.3389/fimmu.2018.02399
A Natural Variant of the Signaling Molecule Vav1 Enhances Susceptibility to Myasthenia Gravis and Influences the T Cell Receptor Repertoire
Abstract
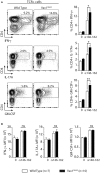
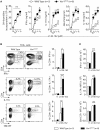
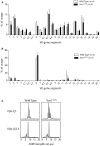
The guanine nucleotide exchange factor Vav1 is essential for transducing T cell receptor (TCR) signals and plays an important role in T cell development and activation. Previous genetic studies identified a natural variant of Vav1 characterized by the substitution of an arginine (R) residue by a tryptophane (W) at position 63 (Vav1R63W). This variant impacts Vav1 adaptor functions and controls susceptibility to T cell-mediated neuroinflammation. To assess the implication of this Vav1 variant on the susceptibility to antibody-mediated diseases, we used the animal model of myasthenia gravis, experimental autoimmune myasthenia gravis (EAMG). To this end, we generated a knock-in (KI) mouse model bearing a R to W substitution in the Vav1 gene (Vav1R63W) and immunized it with either torpedo acetylcholine receptor (tAChR) or the α146-162 immunodominant peptide. We observed that the Vav1R63W conferred increased susceptibility to EAMG, revealed by a higher AChR loss together with an increased production of effector cytokines (IFN-γ, IL-17A, GM-CSF) by antigen-specific CD4+ T cells, as well as an increased frequency of antigen-specific CD4+ T cells. This correlated with the emergence of a dominant antigen-specific T cell clone in KI mice that was not present in wild-type mice, suggesting an impact on thymic selection and/or a different clonal selection threshold following antigen encounter. Our results highlight the key role of Vav1 in the pathophysiology of EAMG and this was associated with an impact on the TCR repertoire of AChR reactive T lymphocytes.
Keywords: T cell repertoire; T cells; Vav1; animal models; myasthenia gravis.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

