Impact of Disease-Modifying Treatments on the Longitudinal Evolution of Anti-JCV Antibody Index in Multiple Sclerosis
- PMID: 30410486
- PMCID: PMC6209669
- DOI: 10.3389/fimmu.2018.02435
Impact of Disease-Modifying Treatments on the Longitudinal Evolution of Anti-JCV Antibody Index in Multiple Sclerosis
Abstract
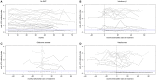
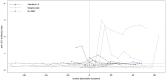
Background: Risk of natalizumab-related progressive multifocal leukoencephalopathy is associated with the presence of anti-JC-virus (JCV) antibodies. Objective: To investigate the impact of disease-modifying treatments (DMT) on the longitudinal evolution of anti-JCV antibody index. Methods: Patients with multiple sclerosis who had serum sampling at intervals of 6 ± 3 months over up to 6 years and who either started DMT (interferon-β, glatiramer acetate or natalizumab) during the observation period with at least one serum sample available before and after treatment initiation or received no DMT during the observation period were included. Anti-JCV antibody serological status and index were determined by 2-step second-generation anti-JCV antibody assay. Results: A total of 89 patients were followed for a median time of 55.2 months. Of those, 62 (69.7%) started DMT and 27 (30.3%) were without therapy during the observation period. Variation of longitudinal anti-JCV antibody index ranged from 9 to 15% and was similar in patients with and without DMT. Applying a mixed model considering the combined effects of treatment and time as well as individual heterogeneity did not show a significant change of anti-JCV antibody index by the start of treatment with interferon-β, glatiramer acetate, or natalizumab. Conclusion: Evaluated DMTs do not impact longitudinal anti-JCV antibody index evolution.
Keywords: JC virus; anti-JCV antibody index; glatiramer acetate; interferon beta; longitudinal; multiple sclerosis; natalizumab; seroconversion.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

