Ivermectin Promotes Peripheral Nerve Regeneration during Wound Healing
- PMID: 30411007
- PMCID: PMC6210064
- DOI: 10.1021/acsomega.8b01451
Ivermectin Promotes Peripheral Nerve Regeneration during Wound Healing
Abstract
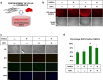
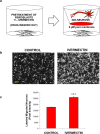
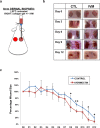
Peripheral nerves have the capacity to regenerate due to the presence of neuroprotective glia of the peripheral nervous system, Schwann cells. Upon peripheral nerve injury, Schwann cells create a permissive microenvironment for neuronal regrowth by taking up cytotoxic glutamate and secreting neurotrophic signaling molecules. Impaired peripheral nerve repair is often caused by a defective Schwann cell response after injury, and there is a critical need to develop new strategies to enhance nerve regeneration, especially in organisms with restricted regenerative potential, such as humans. One approach is to explore mechanisms in lower organisms, in which nerve repair is much more efficient. A recent study demonstrated that the antiparasitic drug, ivermectin, caused hyperinnervation of primordial eye tissue in Xenopus laevis tadpoles. Our study aimed to examine the role of ivermectin in mammalian nerve repair. We performed in vitro assays utilizing human induced neural stem cells (hiNSCs) in co-culture with human dermal fibroblasts (hDFs) and found that ivermectin-treated hDFs promote hiNSC proliferation and migration. We also characterized the effects of ivermectin on hDFs and found that ivermectin causes hDFs to uptake extracellular glutamate, secrete glial cell-derived neurotrophic factor, develop an elongated bipolar morphology, and express glial fibrillary acidic protein. Finally, in a corresponding in vivo model, we found that localized ivermectin treatment in a dermal wound site induced the upregulation of both glial and neuronal markers upon healing. Taken together, we demonstrate that ivermectin promotes peripheral nerve regeneration by inducing fibroblasts to adopt a glia-like phenotype.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Fu S. Y.; Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J. Neurosci. 1995, 15, 3886–3895. 10.1523/JNEUROSCI.15-05-03886.1995. - DOI - PMC - PubMed
- Painter M. W.; Brosius Lutz A.; Cheng Y. C.; Latremoliere A.; Duong K.; Miller C. M.; Posada S.; Cobos E. J.; Zhang A. X.; Wagers A. J.; Havton L. A.; Barres B.; Omura T.; Woolf C. J. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 2014, 83, 331–343. 10.1016/j.neuron.2014.06.016. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

