Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats
- PMID: 30411013
- PMCID: PMC6217532
- DOI: 10.1021/acsomega.8b02015
Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats
Abstract
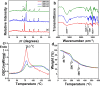
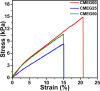
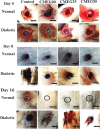
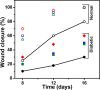
Artificial skin substitute made of polymeric films are of great demand in the field of skin tissue engineering. We report here the fabrication of carboxymethyl cellulose (CMC) and poly(ethylene glycol) (PEG) blend films by solution casting method for wound healing applications. The physicochemical characteristics and the thermal stability of the films were analyzed. The surface morphology shows crystalline structures with large hexagonal-like platelet crystals of CMC on the surface of the films. Pure CMC films exhibited higher tensile strength than the CMC/PEG blend films. The swelling ratio (SR) of the films was influenced by the pH of Tris-HCL buffer (2.0, 5.0, and 7.0), which increased with increase in pH. The hemocompatibility assay and cytotoxicity test using NIH 3T3 fibroblast cells showed that the films were biocompatible. To evaluate the wound healing efficacy, the films were applied in full-thickness wounds created in normal and diabetic Wistar albino rats. The wounds healed faster with pure CMC film compared to blend films in both normal and diabetic rats, evidenced by intensive collagen formation in histopathological analysis. Thus, the films have potential application in skin regeneration, thereby to restore the structural and functional characteristics of the skin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Microwave-Treated Physically Cross-Linked Sodium Alginate and Sodium Carboxymethyl Cellulose Blend Polymer Film for Open Incision Wound Healing in Diabetic Animals-A Novel Perspective for Skin Tissue Regeneration Application.Pharmaceutics. 2023 Jan 27;15(2):418. doi: 10.3390/pharmaceutics15020418. Pharmaceutics. 2023. PMID: 36839741 Free PMC article.
-
Preparation, Characterization, and Biological Evaluation of Transparent Thin Carboxymethyl-Chitosan/Oxidized Carboxymethyl Cellulose Films as New Wound Dressings.Macromol Biosci. 2022 Feb;22(2):e2100308. doi: 10.1002/mabi.202100308. Epub 2021 Dec 2. Macromol Biosci. 2022. PMID: 34752675
-
Preparation and Characterization of Blended Films from Quaternized Hemicelluloses and Carboxymethyl Cellulose.Materials (Basel). 2015 Dec 23;9(1):4. doi: 10.3390/ma9010004. Materials (Basel). 2015. PMID: 28787804 Free PMC article.
-
Synthesis, characterization and application of gelatin-carboxymethyl cellulose blend films for preservation of cherry tomatoes and grapes.J Food Sci Technol. 2019 Jun;56(6):3099-3108. doi: 10.1007/s13197-019-03809-3. Epub 2019 May 16. J Food Sci Technol. 2019. PMID: 31205364 Free PMC article.
-
Synthesis and in vitro characterizations of porous carboxymethyl cellulose-poly(ethylene oxide) hydrogel film.Biomater Res. 2015 Apr 23;19:12. doi: 10.1186/s40824-015-0033-3. eCollection 2015. Biomater Res. 2015. PMID: 26331082 Free PMC article.
Cited by
-
A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro?Cells. 2020 Jul 6;9(7):1622. doi: 10.3390/cells9071622. Cells. 2020. PMID: 32640572 Free PMC article. Review.
-
Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds.Polymers (Basel). 2022 Feb 14;14(4):724. doi: 10.3390/polym14040724. Polymers (Basel). 2022. PMID: 35215637 Free PMC article. Review.
-
One-Step Preparation of Carboxymethyl Cellulose-Phytic Acid Hydrogels with Potential for Biomedical Applications.Gels. 2022 Oct 12;8(10):647. doi: 10.3390/gels8100647. Gels. 2022. PMID: 36286150 Free PMC article.
-
A green approach to obtain stable and hydrophilic cellulose-based electrospun nanofibrous substrates for sustained release of therapeutic molecules.RSC Adv. 2019 Jul 9;9(37):21288-21301. doi: 10.1039/c9ra03399h. eCollection 2019 Jul 5. RSC Adv. 2019. PMID: 35521346 Free PMC article.
-
Apigenin's Influence on Inflammatory and Epigenetic Responses in Rat Lungs After Radiotherapy.Curr Radiopharm. 2025;18(2):147-157. doi: 10.2174/0118744710336823241011095632. Curr Radiopharm. 2025. PMID: 39444181
References
LinkOut - more resources
Full Text Sources
Research Materials

