Enhanced Cancer Theranostics with Self-Assembled, Multilabeled siRNAs
- PMID: 30411024
- PMCID: PMC6217585
- DOI: 10.1021/acsomega.8b01999
Enhanced Cancer Theranostics with Self-Assembled, Multilabeled siRNAs
Abstract
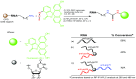
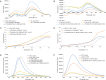
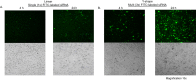
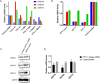
The integration of therapy and diagnostics, termed "theranostics", has recently gained widespread utility in the development of new and improved therapeutics that effectively diagnose and treat diseases, such as cancer. In this study, the covalent attachment of multiple fluorescent labels (i.e., fluorescein isothiocyanate (FITC)) to a wide range of siRNAs, including those adopting linear, V- and Y-shape nanostructures, was successfully accomplished by solid-phase bioconjugation for monitoring cell uptake, co-localization, and biological activity in cell culture. The FITC-labeled higher-order V- and Y-shape siRNAs maintained the requisite hybrid stabilities and A-type helical structures for invoking RNAi activity. The FITC-siRNA hybrids with sense-strand modifiers enabled efficient mRNA knockdown (∼50-90%), which also translated to increased cell death (∼20-95%) in a bone metastatic prostate cancer cell line, over a 72 h incubation period. Significantly, the Y-shaped siRNA containing three FITC probes enhanced fluorescent signaling relative to the siRNA constructs containing single and double fluorophores while retaining potent knockdown and cell death effects post-transfection. Taken together, this data highlights the theranostic utility of the multilabeled FITC-siRNA constructs for potential cancer gene therapy applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Ajith T. A. Strategies used in the clinical trials of gene therapy for cancer. J. Exp. Ther. Oncol. 2017, 11, 33–39. - PubMed
-
- Adams D.et al. Patisiran, an Investigational RNAi Therapeutic for Patients with Hereditary Transthyretin-Mediated (hATTR) Amyloidosis with Polyneuropathy: Results from the Phase 3 APOLLO study (CT.001). Neurology 2018, 90.
LinkOut - more resources
Full Text Sources

