Brain tumour post-treatment imaging and treatment-related complications
- PMID: 30411280
- PMCID: PMC6269328
- DOI: 10.1007/s13244-018-0661-y
Brain tumour post-treatment imaging and treatment-related complications
Abstract
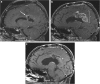
Purpose: The imaging of primary and metastatic brain tumours is very complex and relies heavily on advanced magnetic resonance imaging (MRI). Utilisation of these advanced imaging techniques is essential in helping clinicians determine tumour response after initiation of treatment. Many options are currently available to treat brain tumours, and each can significantly alter the brain tumour appearance on post-treatment imaging. In addition, there are several common and uncommon treatment-related complications that are important to identify on standard post-treatment imaging.
Methods: This article provides a review of the various post-treatment-related imaging appearances of brain neoplasms, including a discussion of advanced MR imaging techniques available and treatment response criteria most commonly used in clinical practice. This article also provides a review of the multitude of treatment-related complications that can be identified on routine post-treatment imaging, with an emphasis on radiation-induced, chemotherapy-induced, and post-surgical entities. Although radiological evaluation of brain tumours after treatment can be quite challenging, knowledge of the various imaging techniques available can help the radiologist distinguish treatment response from tumour progression and has the potential to save patients from inappropriate alterations in treatment. In addition, knowledge of common post-treatment-related complications that can be identified on imaging can help the radiologist play a key role in preventing significant patient morbidity/mortality.
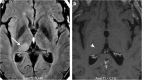
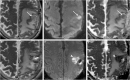
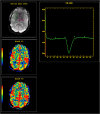
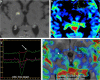
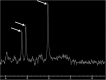
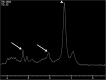
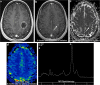
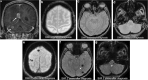
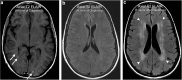
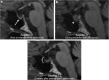
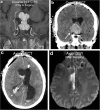
Teaching points: • Contrast enhancement does not reliably define tumour extent in many low-grade or infiltrative gliomas. • Focal regions of elevated cerebral blood volume (rCBV) on dynamic susceptibility contrast (DSC) perfusion-weighted imaging are suggestive of tumour growth/recurrence. • Brain tumour treatment response criteria rely on both imaging and clinical parameters. • Chemotherapeutic agents can potentiate many forms of radiation-induced injury. • Ipilimumab-induced hypophysitis results in transient diffuse enlargement of the pituitary gland.
Keywords: Brain neoplasms; Glioma; Neoplasm metastasis; Radiotherapy; Review.
Figures






References
-
- Bulakbasi N, Guvenc I, Onguru O, Erdogan E, Tayfun C, Ucoz T. The added value of the apparent diffusion coefficient calculation to magnetic resonance imaging in the differentiation and grading of malignant brain tumors. J Comput Assist Tomogr. 2004;28:735–746. doi: 10.1097/00004728-200411000-00003. - DOI - PubMed
-
- Chan AA, Nelson SJ (2004) Simplified gamma-variate fitting of perfusion curves. 2nd IEEE International Symposium on Biomedical Imaging: Nano to Macro 2(2):1067-1070
Publication types
LinkOut - more resources
Full Text Sources

