Inhibitors in haemophilia A and B: Management of bleeds, inhibitor eradication and strategies for difficult-to-treat patients
- PMID: 30411401
- PMCID: PMC6936224
- DOI: 10.1111/ejh.13193
Inhibitors in haemophilia A and B: Management of bleeds, inhibitor eradication and strategies for difficult-to-treat patients
Abstract
The standard therapy for patients with haemophilia is prophylactic treatment with replacement factor VIII (FVIII) or factor IX (FIX). Patients who develop inhibitors against FVIII/FIX face an increased risk of bleeding, and the likelihood of early development of progressive arthropathy, alongside higher treatment-related costs. Bypassing agents can be used to prevent and control bleeding, as well as the recently licensed prophylaxis, emicizumab, but their efficacy is less predictable than that of factor replacement therapy. Antibody eradication, by way of immune tolerance induction (ITI), is still the preferred management strategy for treating patients with inhibitors. This approach is successful in most patients, but some are difficult to tolerise and/or are unresponsive to ITI, and they represent the most complicated patients to treat. However, there are limited clinical data and guidelines available to help guide physicians in formulating the next treatment steps in these patients. This review summarises currently available treatment options for patients with inhibitors, focussing on ITI regimens and those ITI strategies that may be used in difficult-to-treat patients. Some alternative, non-ITI approaches for inhibitor management, are also proposed.
Keywords: coagulation disorders; paediatric haematology; quality of life.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
RL has received consultancy or speaker fees from Shire/Baxter, Bayer, Biogen, CSL Behring, Pfizer, Novo Nordisk and Octapharma. GA has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or consulting, and/or funds for research from Baxter (Baxalta), Bayer HealthCare, Biotest, CSL Behring, Grifols, Novo Nordisk and Pfizer. GB has received honoraria from Novo Nordisk for speaking and participating on advisory boards. GD has received honoraria from Novo Nordisk for speaking and participating on advisory boards. AD received honoraria payment from Novo Nordisk for reviewing research grant applications in 2016. CH has acted as a consultant and been a board member for Shire, Bayer, CAF‐DCF, CSL Behring, LFB, Octapharma, Novo Nordisk, Pfizer, Sobi Biogen and Roche and has received grants from Shire, Bayer and Pfizer. VJ‐Y has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or consulting and/or funds for research from Baxter, Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma and Pfizer. TL has received reimbursement for attending symposia/congresses and/or honoraria for consulting and/or funds for research from Baxter (Baxalta), Bayer, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche and Sobi. MM has acted as a paid consultant to Baxter, Bayer and Novo Nordisk and has served as a consultant on Pfizer advisory boards. He has received speaker fees from Bayer, CSL Behring, Novo Nordisk and Octapharma and has received unrestricted research grants from Baxter, Bayer and Pfizer. SZ‐S has received reimbursement for attending symposia and congresses, and honoraria payment for speaking, from Baxter, Novo Nordisk, Sobi Biogen and Octapharma. ES has received speaker fees for meetings organised by Bayer, Shire, Sobi, Bioverativ, CSL Behring, Grifols, Kedrion, Novo Nordisk, Octapharma, Roche and Pfizer; acted as paid consultant for Bayer, Shire, Sobi, Bioverativ, CSL Behring, Grifols, Kedrion, Novo Nordisk, Roche and Pfizer.
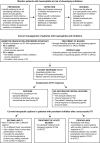
Figures
References
-
- World Federation of Hemophilia . WFH guidelines for the management of hemophilia. 2014. http://www1.wfh.org/publications/files/pdf-1512.pdf. Accessed May 24, 2016
-
- DiMichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007;13(Suppl 1):1‐22. - PubMed
-
- Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124:3365‐3372. - PubMed
-
- Morfini M, Haya S, Tagariello G, et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia. 2007;13:606‐612. - PubMed
-
- Gringeri A, Mantovani LG, Scalone L, Mannucci PM. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 2003;102:2358‐2363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

