Antiretroviral resistance testing in HIV-positive people
- PMID: 30411789
- PMCID: PMC6517236
- DOI: 10.1002/14651858.CD006495.pub5
Antiretroviral resistance testing in HIV-positive people
Abstract
Background: Resistance to antiretroviral therapy (ART) among people living with human immunodeficiency virus (HIV) compromises treatment effectiveness, often leading to virological failure and mortality. Antiretroviral drug resistance tests may be used at the time of initiation of therapy, or when treatment failure occurs, to inform the choice of ART regimen. Resistance tests (genotypic or phenotypic) are widely used in high-income countries, but not in resource-limited settings. This systematic review summarizes the relative merits of resistance testing in treatment-naive and treatment-exposed people living with HIV.
Objectives: To evaluate the effectiveness of antiretroviral resistance testing (genotypic or phenotypic) in reducing mortality and morbidity in HIV-positive people.
Search methods: We attempted to identify all relevant studies, regardless of language or publication status, through searches of electronic databases and conference proceedings up to 26 January 2018. We searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), and ClinicalTrials.gov to 26 January 2018. We searched Latin American and Caribbean Health Sciences Literature (LILACS) and the Web of Science for publications from 1996 to 26 January 2018.
Selection criteria: We included all randomized controlled trials (RCTs) and observational studies that compared resistance testing to no resistance testing in people with HIV irrespective of their exposure to ART.Primary outcomes of interest were mortality and virological failure. Secondary outcomes were change in mean CD4-T-lymphocyte count, clinical progression to AIDS, development of a second or new opportunistic infection, change in viral load, and quality of life.
Data collection and analysis: Two review authors independently assessed each reference for prespecified inclusion criteria. Two review authors then independently extracted data from each included study using a standardized data extraction form. We analysed data on an intention-to-treat basis using a random-effects model. We performed subgroup analyses for the type of resistance test used (phenotypic or genotypic), use of expert advice to interpret resistance tests, and age (children and adolescents versus adults). We followed standard Cochrane methodological procedures.
Main results: Eleven RCTs (published between 1999 and 2006), which included 2531 participants, met our inclusion criteria. All of these trials exclusively enrolled patients who had previous exposure to ART. We found no observational studies. Length of follow-up time, study settings, and types of resistance testing varied greatly. Follow-up ranged from 12 to 150 weeks. All studies were conducted in Europe, USA, or South America. Seven studies used genotypic testing, two used phenotypic testing, and two used both phenotypic and genotypic testing. Only one study was funded by a manufacturer of resistance tests.Resistance testing made little or no difference in mortality (odds ratio (OR) 0.89, 95% confidence interval (CI) 0.36 to 2.22; 5 trials, 1140 participants; moderate-certainty evidence), and may have slightly reduced the number of people with virological failure (OR 0.70, 95% CI 0.56 to 0.87; 10 trials, 1728 participants; low-certainty evidence); and probably made little or no difference in change in CD4 cell count (mean difference (MD) -1.00 cells/mm³, 95% CI -12.49 to 10.50; 7 trials, 1349 participants; moderate-certainty evidence) or progression to AIDS (OR 0.64, 95% CI 0.31 to 1.29; 3 trials, 809 participants; moderate-certainty evidence). Resistance testing made little or no difference in adverse events (OR 0.89, 95% CI 0.51 to 1.55; 4 trials, 808 participants; low-certainty evidence) and probably reduced viral load (MD -0.23, 95% CI -0.35 to -0.11; 10 trials, 1837 participants; moderate-certainty evidence). No studies reported on development of new opportunistic infections or quality of life. We found no statistically significant heterogeneity for any outcomes, and the I² statistic value ranged from 0 to 25%. We found no subgroup effects for types of resistance testing (genotypic versus phenotypic), the addition of expert advice to interpretation of resistance tests, or age. Results for mortality were consistent when we compared studies at high or unclear risk of bias versus studies at low risk of bias.
Authors' conclusions: Resistance testing probably improved virological outcomes in people who have had virological failure in trials conducted 12 or more years ago. We found no evidence in treatment-naive people. Resistance testing did not demonstrate important patient benefits in terms of risk of death or progression to AIDS. The trials included very few participants from low- and middle-income countries.
Conflict of interest statement
JT has no known conflicts of interest. TA has no known conflicts of interest. RS has no known conflicts of interest. LM has no known conflicts of interest.
Figures

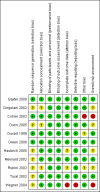
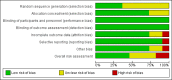






Update of
- doi: 10.1002/14651858.CD006495.pub4
References
References to studies included in this review
Baxter 2000 {published data only}
-
- Baxter JD, Mayers DL, Wentworth DN, Neaton JD, Hoover ML, Winters MA, et al. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. CPCRA 046 Study Team for the Terry Beirn Community Programs for Clinical Research on AIDS. AIDS (London, England) 2000;14(9):F83‐93. [PUBMED: 10894268] - PubMed
Cingolani 2002 {published data only}
-
- Cingolani A, Antinori A, Rizzo MG, Murri R, Ammassari A, Baldini F, et al. Usefulness of monitoring HIV drug resistance and adherence in individuals failing highly active antiretroviral therapy: a randomized study (ARGENTA). AIDS (London, England) 2002;16(3):369‐79. [PUBMED: 11834948] - PubMed
Cohen 2002 {published data only}
-
- Cohen CJ, Hunt S, Sension M, Farthing C, Conant M, Jacobson S, et al. A randomized trial assessing the impact of phenotypic resistance testing on antiretroviral therapy. AIDS (London, England) 2002;16(4):579‐88. [PUBMED: 11873001] - PubMed
Dunn 2005 {published data only}
-
- Evaluation of Resistance Assays (ERA) Trial Investigators. A randomized controlled trial of the clinical utility of genotypic resistance testing in patients with limited prior exposure to antiretroviral drugs. HIV Clinical Trials 2005;6(4):183‐6. [PUBMED: 16214734] - PubMed
Durant 1999 {published data only}
-
- Durant J, Clevenbergh P, Halfon P, Delgiudice P, Porsin S, Simonet P, et al. Drug‐resistance genotyping in HIV‐1 therapy: the VIRADAPT randomised controlled trial. Lancet (London, England) 1999;353(9171):2195‐9. [PUBMED: 10392984] - PubMed
Green 2006 {published data only}
-
- Green H, Gibb DM, Compagnucci A, Giacomet V, Rossi A, Harper L, et al. A randomized controlled trial of genotypic HIV drug resistance testing in HIV‐1‐infected children: the PERA (PENTA 8) trial. Antiviral Therapy 2006;11(7):857‐67. [PUBMED: 17302248] - PubMed
Haubrich 2005 {published data only}
-
- Haubrich RH, Kemper CA, Hellmann NS, Keiser PH, Witt MD, Tilles JG, et al. A randomized, prospective study of phenotype susceptibility testing versus standard of care to manage antiretroviral therapy: CCTG 575. AIDS (London, England) 2005;19(3):295‐302. [PUBMED: 15718840] - PubMed
Meynard 2002 {published data only}
-
- Meynard JL, Vray M, Morand‐Joubert L, Race E, Descamps D, Peytavin G, et al. Phenotypic or genotypic resistance testing for choosing antiretroviral therapy after treatment failure: a randomized trial. AIDS (London, England) 2002;16(5):727‐36. [PUBMED: 11964529] - PubMed
Rubini 2002 {unpublished data only}
-
- Rubini NPM, Brindeiro PA, Brindeiro RM, Llerena CA, Hertogs K, Tanuri A. Clinical value of HIV‐1 genotyping in children and adolescents heavily experiences in antiretroviral therapy. XIV International AIDS Conference. Barcelona, 2002.
Tural 2002 {published data only}
-
- Tural C, Ruiz L, Holtzer C, Schapiro J, Viciana P, Gonzalez J, et al. Clinical utility of HIV‐1 genotyping and expert advice: the Havana trial. AIDS (London, England) 2002;16(2):209‐18. [PUBMED: 11807305] - PubMed
Wegner 2004 {published data only}
-
- Wegner SA, Wallace MR, Aronson NE, Tasker SA, Blazes DL, Tamminga C, et al. Long‐term efficacy of routine access to antiretroviral‐resistance testing in HIV type 1‐infected patients: results of the clinical efficacy of resistance testing trial. Clinical Infectious Diseases 2004;38(5):723‐30. [PUBMED: 14986258] - PubMed
References to studies excluded from this review
Badri 2003 {published data only}
-
- Badri SM, Adeyemi OM, Max BE, Zagorski BM, Barker DE. How does expert advice impact genotypic resistance testing in clinical practice?. Clinical Infectious Diseases 2003;37:708‐13. - PubMed
Bonjoch 2008 {published data only}
-
- Bonjoch A, Buzon MJ, Llibre JM, Negredo E, Puig J, Perez‐Alvarez N, et al. Transient treatment exclusively containing nucleoside analogue reverse transcriptase inhibitors in highly antiretroviral‐experienced patients preserves viral benefit when a fully active therapy was initiated. HIV Clinical Trials 2008;9:387‐98. - PubMed
Bossi 2004 {published data only}
-
- Bossi P, Peytavin G, Ait‐Mohand H, Delaugerre C, Ktorza N, Paris L, et al. GENOPHAR: a randomized study of plasma drug measurements in association with genotypic resistance testing and expert advice to optimize therapy in patients failing antiretroviral therapy. HIV Medicine 2004;5:352‐9. - PubMed
Clevenbergh 2000 {published data only}
-
- Clevenbergh P, Durant J, Halfon P, Giudice P, Mondain V, Montagne N, et al. Persisting long‐term benefit of genotype‐guided treatment for HIV‐infected patients failing HAART. The Viradapt Study: week 48 follow‐up. Antiviral Therapy 2000;5:65‐70. - PubMed
Clevenbergh 2002 {published data only}
-
- Clevenbergh P, Garraffo R, Durant J, Dellamonica P. PharmAdapt: a randomized prospective study to evaluate the benefit of therapeutic monitoring of protease inhibitors: 12 week results. AIDS 2002;16:2311‐5. - PubMed
De Luca 2006 {published data only}
-
- Luca A, Giambenedetto S, Cingolani A, Bacarelli A, Ammassari A, Cauda R. Three‐year clinical outcomes of resistance genotyping and expert advice: extended follow‐up of the Argenta trial. Antiviral Therapy 2006;11:321‐7. - PubMed
Demeter 2008 {published data only}
-
- Demeter LM, Mukherjee AL, DiFrancesco R, Jiang H, DiCenzo R, Bastow B, et al. The design and implementation of A5146, a prospective trial assessing the utility of therapeutic drug monitoring using an inhibitory quotient in antiretroviral‐experienced HIV‐infected patients. HIV Clinical Trials 2008;9:61‐72. - PMC - PubMed
Dunn 2005a {published data only}
-
- Dunn D. A randomized controlled trial of the value of phenotypic testing in addition to genotypic testing for HIV drug resistance. Journal of Acquired Immune Deficiency Syndromes 2005;38:553‐9. - PubMed
Durant 2000 {published data only}
-
- Durant J, Clevenbergh P, Garraffo R, Halfon P, Icard S, Giudice P, et al. Importance of protease inhibitor plasma levels in HIV‐infected patients treated with genotypic‐guided therapy: pharmacological data from the Viradapt Study. AIDS 2000;14:1333‐9. - PubMed
Gianotti 2006 {published data only}
-
- Gianotti N, Mondino V, Rossi MC, Chiesa E, Mezzaroma I, Ladisa N, et al. Comparison of a rule‐based algorithm with a phenotype‐based algorithm for the interpretation of HIV genotypes in guiding salvage regimens in HIV‐infected patients by a randomized clinical trial: the mutations and salvage study. Clinical Infectious Diseases 2006; Vol. 42:1470‐80. - PubMed
Hales 2006 {published data only}
Lorenzana 2012 {published data only}
Maggiolo 2007 {published data only}
-
- Maggiolo F, Airoldi M, Callegaro A, Ripamonti D, Gregis G, Quinzan G, et al. Prediction of virologic outcome of salvage antiretroviral treatment by different systems for interpreting genotypic HIV drug resistance. Journal of the International Association of Physicians in AIDS Care 2007;6:87‐93. - PubMed
Mazzotta 2003 {published data only}
-
- Mazzotta F, Lo Caputo S, Torti C, Tinelli C, Pierotti P, Castelli F, et al. Real versus virtual phenotype to guide treatment in heavily pretreated patients: 48‐week follow‐up of the Genotipo‐Fenotipo di Resistenza (GenPheRex) trial. Journal of Acquired Immune Deficiency Syndromes 2003;32:268‐80. - PubMed
Pere 2012 {published data only}
-
- Pere H, Charpentier C, Mbelesso P, Dandy M, Matta M, Moussa S, et al. Virological response and resistance profiles after 24 months of first‐line antiretroviral treatment in adults living in Bangui, central African Republic. AIDS Research and Human Retroviruses 2012;28:315‐23. - PubMed
Perez‐Elias 2003 {published data only}
-
- Perez‐Elias MJ, Garcia‐Arata I, Munoz V, Santos I, Sanz J, Abraira V, et al. Phenotype or virtual phenotype for choosing antiretroviral therapy after failure: a prospective, randomized study. Antiviral Therapy 2003;8:577‐84. - PubMed
Saracino 2004 {published data only}
-
- Saracino A, Monno L, Locaputo S, Torti C, Scudeller L, Ladisa N, et al. Selection of antiretroviral therapy guided by genotypic or phenotypic resistance testing: an open‐label, randomized, multicenter study (PhenGen). Journal of Acquired Immune Deficiency Syndromes 2004;37:1587‐98. - PubMed
Torti 2005 {published data only}
-
- Torti C, Quiros‐Roldan E, Regazzi M, Luca A, Mazzotta F, Antinori A, et al. A randomized controlled trial to evaluate antiretroviral salvage therapy guided by rules‐based or phenotype‐driven HIV‐1 genotypic drug‐resistance interpretation with or without concentration‐controlled intervention: the resistance and dosage adapted regimens (RADAR) study. Clinical Infectious Diseases 2005;40:1828‐36. - PubMed
Vergne 2003 {published data only}
-
- Vergne L, Kane CT, Laurent C, Diakhate N, Ngom Gueye NF, Gueye PM, et al. Low rate of genotypic HIV‐1 drug‐resistant strains in the Senegalese government initiative of access to antiretroviral therapy. AIDS 2003;17:S31‐8. - PubMed
Additional references
AIDSinfo 2016
-
- AIDSinfo. Guidelines for the Use of Antiretroviral Agents in HIV‐1‐Infected Adults and Adolescents. Laboratory Testing. Last Updated: 14 July 2016. http://aidsinfo.nih.gov/guidelines/html/1/adult‐and‐adolescent‐arv‐guide... (accessed 03 June 2017).
Bakhouch 2009
-
- Bakhouch K, Oulad‐Lahcen A, Bensghir R, Blaghen M, Elfilali KM, Ezzikouri S, et al. The prevalence of resistance‐associated mutations to protease and reverse transcriptase inhibitors in treatment‐naïve (HIV1)‐infected individuals in Casablanca, Morocco. Journal of Infection in Developing Countries 2009;3(5):380‐91. - PubMed
Barennes 2014
-
- Barennes H, Guillet S, Limsreng S, Him S, Nouhin J, Hak C, et al. Virological failure and HIV‐1 drug resistance mutations among naive and antiretroviral pre‐treated patients entering the ESTHER program of Calmette Hospital in Cambodia. PLoS ONE 2014;9(8):e105736. [DOI: 10.1371/journal.pone.0105736] - DOI - PMC - PubMed
Barrow 2013
Bissio 2017
-
- Bissio E, Barbas MG, Bouzas MB, Cudolá A, Salomón H, Espínola L, et al. Pretreatment HIV‐1 drug resistance in Argentina: results from a surveillance study performed according to WHO‐proposed new methodology in 2014‐15. Journal of Antimicrobial Chemotherapy 2017;72(2):504‐10. [PUBMED: 27789684] - PubMed
Boender 2015
-
- Boender TS, Hoenderboom BM, Sigaloff KC, Hamers RL, Wellington M, Shamu T, et al. Pretreatment HIV drug resistance increases regimen switches in sub‐Saharan Africa. Clinical Infectious Diseases 2015;61(11):1749‐58. [PUBMED: 26240203] - PubMed
Boender 2016
-
- Boender TS, Kityo CM, Boerma RS, Hamers RL, Ondoa P, Wellington M, et al. Accumulation of HIV‐1 drug resistance after continued virological failure on first‐line ART in adults and children in sub‐Saharan Africa. Journal of Antimicrobial Chemotherapy 2016;71(10):2918‐27. [PUBMED: 27342546] - PubMed
Burchell 2012
-
- Burchell AN, Bayoumi AM, Rourke SB, Major C, Gardner S, Sandstrom P, et al. Increase in transmitted HIV drug resistance among persons undergoing genotypic resistance testing in Ontario, Canada, 2002‐09. Journal of Antimicrobial Chemotherapy 2012;67:2755‐65. - PubMed
Cambiano 2013
-
- Cambiano V, Bertagnolio S, Jordan MR, Lundgren JD, Phillips A. Transmission of drug resistant HIV and its potential impact on mortality and treatment outcomes in resource‐limited settings. Journal of Infectious Diseases 2013;207(Suppl 2):S57‐62. [PUBMED: 23687290] - PubMed
Chaiyachati 2014
-
- Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS (London, England) 2014;28(Suppl 2):S187‐204. [PUBMED: 24849479] - PubMed
Corzillius 2004
-
- Corzillius M, Mühlberger N, Sroczynski G, Jaeger H, Wasem J, Siebert U. Cost effectiveness analysis of routine use of genotypic antiretroviral resistance testing after failure of antiretroviral treatment for HIV. Antiviral Therapy 2004;9(1):27‐36. [PUBMED: 15040534] - PubMed
De Luca 2015
Desai 2007
-
- Desai S, Kyriakides T, Holodniy M, Al‐Salman J, Griffith B, Kozal M. Evolution of genotypic resistance algorithms and their impact on the interpretation of clinical trials: an OPTIMA trial substudy. HIV Clinical Trials 2007;8(5):293‐302. [PUBMED: 17956830] - PubMed
Dettori 2011
Dunn 2004
-
- Dunn DT, Gibb DM, Babiker AG, Green H, Darbyshire JH, Weller IV. HIV drug resistance testing: is the evidence really there?. Antiviral Therapy 2004;9(5):641‐8. [PUBMED: 15535402] - PubMed
Duwe 2001
-
- Duwe S, Brunn M, Altmann D, Hamouda O, Schmidt B, Walter H, et al. Frequency of genotypic and phenotypic drug resistant HIV‐1 among therapy‐naive patients of the German Seroconverter Study. Journal of Acquired Immune Deficiency Syndromes 2001;26(3):266‐73. [PUBMED: 11242200] - PubMed
EACS 2017
-
- European AIDS Clinical Society. Guidelines 2017. www.eacsociety.org/files/guidelines_9.0‐english.pdf (accessed 2 August 2018).
Egger 1997
Ena 2006
-
- Ena J, Ruiz de Apodaca RF, Amador C, Benito C, Pasquau F. Net benefits of resistance testing directed therapy compared with standard of care in HIV‐infected patients with virological failure: a meta‐analysis. Enfermedades Infecciosas y Microbiologia Clinica 2006;24(4):232‐7. [PUBMED: 16725082] - PubMed
GRADEpro 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 3 June 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gupta 2012
-
- Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, et al. Global trends in antiretroviral resistance in treatment‐naive individuals with HIV after rollout of antiretroviral treatment in resource‐limited settings: a global collaborative study and meta‐regression analysis. Lancet (London, England) 2012;380(9849):1250‐8. [PUBMED: 22828485] - PMC - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Hamers 2012
-
- Hamers RL, Schuurman R, Sigaloff KC, Wallis CL, Kityo C, Siwale M, et al. Effect of pretreatment HIV‐1 drug resistance on immunological, virological, and drug‐resistance outcomes of first‐line antiretroviral treatment in sub‐Saharan Africa: a multicentre cohort study. Lancet Infectious Diseases 2012;12(4):307‐17. [PUBMED: 22036233] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Version 5.0.2 [updated 2009]. The Cochrane Collaboration.
Hosseinipour 2013
Hozo 2005
Kanters 2017
-
- Kanters S, Park JJ, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta‐analysis. Lancet HIV 2017;4(1):e31‐40. [PUBMED: 27863996] - PubMed
Larder 1989
-
- Larder BA, Richman DD. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science 1989;243(4899):1731‐4. [PUBMED: 2467383] - PubMed
Moher 2010
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. International Journal of Surgery (London, England) 2010;8(5):336‐41. [PUBMED: 20171303] - PubMed
Nachega 2012
-
- Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low‐income, middle‐income, and high‐income countries: a systematic review and meta‐analysis. AIDS (London, England) 2012;26(16):2039‐52. [PUBMED: 22951634] - PMC - PubMed
Palella 2009
-
- Palella FJ Jr, Armon C, Buchacz K, Cole SR, Chmiel JS, Novak RM, et al. The association of HIV susceptibility testing with survival among HIV‐infected patients receiving antiretroviral therapy: a cohort study. Annals of Internal Medicine 2009;151(2):73‐84. [PUBMED: 19620160] - PubMed
Panidou 2004
-
- Panidou ET, Trikalinos TA, Ioannidis JP. Limited benefit of antiretroviral resistance testing in treatment‐experienced patients: a meta‐analysis. AIDS (London, England) 2004;18(16):2153‐61. [PUBMED: 15577648] - PubMed
Pham 2014
Phillips 2014
Pinoges 2015
-
- Pinoges L, Schramm B, Poulet E, Balkan S, Szumilin E, Ferreyra C, et al. Risk factors and mortality associated with resistance to first‐line antiretroviral therapy: multicentric cross‐sectional and longitudinal analyses. Journal of Acquired Immune Deficiency Syndromes 2015;68(5):527‐35. [PUBMED: 25585301] - PubMed
Ramjan 2014
-
- Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, et al. Systematic review and meta‐analysis: patient and programme impact of fixed‐dose combination antiretroviral therapy. Tropical Medicine & International Health 2014;19(5):501‐13. [PUBMED: 24628918] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rocheleau 2017
Rojas Sánchez 2014
-
- Rojas Sánchez P, Holguín A. Drug resistance in the HIV‐1‐infected paediatric population worldwide: a systematic review. Journal of Antimicrobial Chemotherapy 2014;69(8):2032‐42. [PUBMED: 24788658] - PubMed
Rowley 2016
-
- Rowley CF, MacLeod IJ, Maruapula D, Lekoko B, Gaseitsiwe S, Mine M, et al. Sharp increase in rates of HIV transmitted drug resistance at antenatal clinics in Botswana demonstrates the need for routine surveillance. Journal of Antimicrobial Chemotherapy 2016;71(5):1361‐6. [PUBMED: 26929269] - PMC - PubMed
Saag 2018
Sax 2005
-
- Sax PE, Islam R, Walensky RP, Losina E, Weinstein MC, Goldie SJ, et al. Should resistance testing be performed for treatment‐naive HIV‐infected patients? A cost‐effectiveness analysis. Clinical Infectious Diseases 2005;41(9):1316‐23. [PUBMED: 16206108] - PubMed
Sluis‐Cremer 2014
Sullivan 2013
-
- Sullivan A, Sutcliffe P, Sandre R, Harrigan R, Archibald C, Halverson J, et al. Follow‐up investigation of a cluster of treatment‐naive HIV‐infected patients with multi‐drug resistance in Sudbury, Ontario. Canadian Journal of Infectious Diseases and Medical Microbiology 2013;24:38A.
Thompson 2012
-
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence‐based recommendations from an International Association of Physicians in AIDS Care Panel. Annals of Internal Medicine 2012;156(11):817‐33, W‐284‐94. [PUBMED: 22393036] - PMC - PubMed
Torre 2002
-
- Torre D, Tambini R. Antiretroviral drug resistance testing in patients with HIV‐1 infection: a meta‐analysis study. HIV Clinical Trials 2002;3(1):1‐8. [PUBMED: 11819179] - PubMed
Torti 2004
-
- Torti C, Bono L, Gargiulo F, Uccelli MC, Quiros‐Roldan E, Patroni A, et al. Prevalence of drug resistance and newly recognised treatment‐related substitutions in the HIV‐1 reverse transcriptase and protease genes from HIV‐positive patients naive for antiretrovirals. Clinical Microbiology and Infection 2004;10(9):826‐30. [PUBMED: 15355414] - PubMed
Tsiang 2016
UNAIDS 2014
-
- UNAIDS. The Gap Report. 2014. http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspu... (accessed 3 June 2017).
UNAIDS 2016
-
- UNAIDS. Get on the Fast‐Track. The Life‐Cycle Approach to HIV. Finding Solutions for Everyone at Every Stage of Life. 2016. www.unaids.org/sites/default/files/media_asset/Get‐on‐the‐Fast‐Track_en.pdf (accessed 3 June 2017).
Villabona‐Arenas 2016
-
- Villabona‐Arenas CJ, Vidal N, Guichet E, Serrano L, Delaporte E, Gascuel O, et al. In‐depth analysis of HIV‐1 drug resistance mutations in HIV‐infected individuals failing first‐line regimens in West and Central Africa. AIDS (London, England) 2016;30(17):2577‐89. [PUBMED: 27603287] - PubMed
Wells 2009
-
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2009. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 3 June 2017).
WHO 2016
-
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Second Edition. June 2016. www.who.int/hiv/pub/arv/arv‐2016/en/ (accessed 3 June 2017).
WHO 2017
-
- World Health Organization. Antiretroviral Therapy (ART) Coverage Among All Age Groups. www.who.int/gho/hiv/epidemic_response/ART_text/en/ (accessed 3 June 2017).
WHO 2017a
-
- World Health Organization. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance. http://apps.who.int/iris/bitstream/handle/10665/255880/9789241550055‐eng... (accessed 25 July 2018).
WHO 2017b
-
- World Health Organization. 20th WHO Model List of Essential Medicines. http://www.who.int/medicines/publications/essentialmedicines/20th_EML201... (accessed 24 April 2018).
WHO 2017c
-
- World Health Organization. WHO HIV Drug Resistance Report 2017. http://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831‐eng... (accessed 24 July 2018).
Wittkop 2011
-
- Wittkop L, Günthard HF, Wolf F, Dunn D, Cozzi‐Lepri A, Luca A, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord‐CHAIN joint project): a European multicohort study. Lancet Infectious Diseases 2011;11(5):363‐71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

