Microvascular mechanisms limiting skeletal muscle blood flow with advancing age
- PMID: 30412030
- PMCID: PMC6737458
- DOI: 10.1152/japplphysiol.00113.2018
Microvascular mechanisms limiting skeletal muscle blood flow with advancing age
Abstract
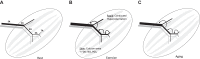
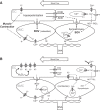
Effective oxygen delivery to active muscle fibers requires that vasodilation initiated in distal arterioles, which control flow distribution and capillary perfusion, ascends the resistance network into proximal arterioles and feed arteries, which govern total blood flow into the muscle. With exercise onset, ascending vasodilation reflects initiation and conduction of hyperpolarization along endothelium from arterioles into feed arteries. Electrical coupling of endothelial cells to smooth muscle cells evokes the rapid component of ascending vasodilation, which is sustained by ensuing release of nitric oxide during elevated luminal shear stress. Concomitant sympathetic neural activation inhibits ascending vasodilation by stimulating α-adrenoreceptors on smooth muscle cells to constrict the resistance vasculature. We hypothesized that compromised muscle blood flow in advanced age reflects impaired ascending vasodilation through actions on both cell layers of the resistance network. In the gluteus maximus muscle of old (24 mo) vs. young (4 mo) male mice (corresponding to mid-60s vs. early 20s in humans) inhibition of α-adrenoreceptors in old mice restored ascending vasodilation, whereas even minimal activation of α-adrenoreceptors in young mice attenuated ascending vasodilation in the manner seen with aging. Conduction of hyperpolarization along the endothelium is impaired in old vs. young mice because of "leaky" membranes resulting from the activation of potassium channels by hydrogen peroxide released from endothelial cells. Exposing the endothelium of young mice to hydrogen peroxide recapitulates this effect of aging. Thus enhanced α-adrenoreceptor activation of smooth muscle in concert with electrically leaky endothelium restricts muscle blood flow by impairing ascending vasodilation in advanced age.
Keywords: adrenoreceptors; aging; ascending vasodilation; endothelium; potassium channels.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

