Inhibition of mTORC1 by ER stress impairs neonatal β-cell expansion and predisposes to diabetes in the Akita mouse
- PMID: 30412050
- PMCID: PMC6294551
- DOI: 10.7554/eLife.38472
Inhibition of mTORC1 by ER stress impairs neonatal β-cell expansion and predisposes to diabetes in the Akita mouse
Abstract
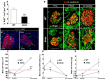
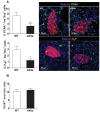
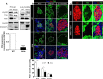
Unresolved ER stress followed by cell death is recognized as the main cause of a multitude of pathologies including neonatal diabetes. A systematic analysis of the mechanisms of β-cell loss and dysfunction in Akita mice, in which a mutation in the proinsulin gene causes a severe form of permanent neonatal diabetes, showed no increase in β-cell apoptosis throughout life. Surprisingly, we found that the main mechanism leading to β-cell dysfunction is marked impairment of β-cell growth during the early postnatal life due to transient inhibition of mTORC1, which governs postnatal β-cell growth and differentiation. Importantly, restoration of mTORC1 activity in neonate β-cells was sufficient to rescue postnatal β-cell growth, and to improve diabetes. We propose a scenario for the development of permanent neonatal diabetes, possibly also common forms of diabetes, where early-life events inducing ER stress affect β-cell mass expansion due to mTOR inhibition.
Keywords: Beta cells; ER stress; diabetes; human biology; mTOR; medicine; mouse; proinsulin misfolding; proliferation.
© 2018, Riahi et al.
Conflict of interest statement
YR, TI, RY, SC, DA, MS, IA, MS, NP, EB, YD, EC, GL No competing interests declared
Figures






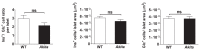


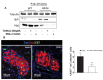



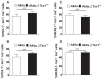

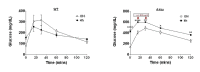
Comment in
-
Folding mutations suppress early beta-cell proliferation.Elife. 2018 Dec 14;7:e43475. doi: 10.7554/eLife.43475. Elife. 2018. PMID: 30547883 Free PMC article.
References
-
- Alejandro EU, Gregg B, Wallen T, Kumusoglu D, Meister D, Chen A, Merrins MJ, Satin LS, Liu M, Arvan P, Bernal-Mizrachi E. Maternal diet-induced microRNAs and mTOR underlie β cell dysfunction in offspring. Journal of Clinical Investigation. 2014;124:4395–4410. doi: 10.1172/JCI74237. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

