Insulin mutations impair beta-cell development in a patient-derived iPSC model of neonatal diabetes
- PMID: 30412052
- PMCID: PMC6294552
- DOI: 10.7554/eLife.38519
Insulin mutations impair beta-cell development in a patient-derived iPSC model of neonatal diabetes
Abstract
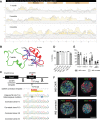
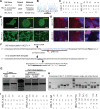
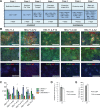
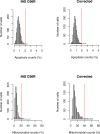
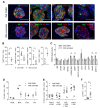
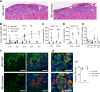
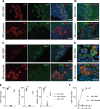
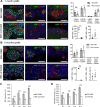
Insulin gene mutations are a leading cause of neonatal diabetes. They can lead to proinsulin misfolding and its retention in endoplasmic reticulum (ER). This results in increased ER-stress suggested to trigger beta-cell apoptosis. In humans, the mechanisms underlying beta-cell failure remain unclear. Here we show that misfolded proinsulin impairs developing beta-cell proliferation without increasing apoptosis. We generated induced pluripotent stem cells (iPSCs) from people carrying insulin (INS) mutations, engineered isogenic CRISPR-Cas9 mutation-corrected lines and differentiated them to beta-like cells. Single-cell RNA-sequencing analysis showed increased ER-stress and reduced proliferation in INS-mutant beta-like cells compared with corrected controls. Upon transplantation into mice, INS-mutant grafts presented reduced insulin secretion and aggravated ER-stress. Cell size, mTORC1 signaling, and respiratory chain subunits expression were all reduced in INS-mutant beta-like cells, yet apoptosis was not increased at any stage. Our results demonstrate that neonatal diabetes-associated INS-mutations lead to defective beta-cell mass expansion, contributing to diabetes development.
Keywords: CRISPR-Cas9; Insulin gene mutations; beta-cell development; endoplasmic reticulum stress; human; induced pluripotent stem cells; mTORC1; regenerative medicine; stem cells.
© 2018, Balboa et al.
Conflict of interest statement
DB, JS, DB, MS, PL, EG, SE, JU, HG, HH, JP, KW, TO No competing interests declared
Figures






Comment in
-
Folding mutations suppress early beta-cell proliferation.Elife. 2018 Dec 14;7:e43475. doi: 10.7554/eLife.43475. Elife. 2018. PMID: 30547883 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

