Efficacy and safety of empagliflozin for type 2 diabetes mellitus: Meta-analysis of randomized controlled trials
- PMID: 30412076
- PMCID: PMC6221554
- DOI: 10.1097/MD.0000000000012843
Efficacy and safety of empagliflozin for type 2 diabetes mellitus: Meta-analysis of randomized controlled trials
Abstract
Background: This study was designed to evaluate the efficiency and tolerability of empagliflozin (EMPA) as monotherapy or add-on to existing therapy in patients with type 2 diabetes mellitus (T2DM).
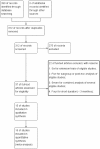
Methods: Randomized controlled trials (RCTs) comparing efficacy and safety of EMPA vs placebo or EMPA plus other antidiabetes drugs vs placebo plus other oral antidiabetes drugs (OADs) in T2DM were recruited from electronic database Pubmed, Web of Knowledge, and Cochrane Central Register of Controlled Trials (CENTRAL), supplemented by a hand search of the reference lists of selected articles. Main effect sizes were change from baseline on glycemia control, body weight, blood pressure, and complications (i.e., incidence of urinary and genital tract infections, and morbidity of hypoglycemia and hyperglycemia). Random-effects model was used to account for clinical or methodologic heterogeneity across studies.
Results: Fifteen RCTs with a total number of 7891 individuals (5374 in EMPA group and 2517 in control group) were suitable for this meta-analysis. The results demonstrated that significant improvements in glycemia control, body weight, and blood pressure were associated with EMPA application (i.e., monotherapy and add-on therapy) in patient with T2DM when compared with placebo. Meanwhile, EMPA 10 and 20 mg improved glycemia, body weight, and blood pressure control for patients with T2DM. There was no significant difference in incidence of hypoglycemia and urinary tract infections across EMPA and placebo group. Significant reduced risk of hyperglycemia was revealed in EMPA group vs placebo (risk ratio: 0.34, 95%confidence interval: 0.23-0.49, P < .00001), except in patients on background insulin therapy. However, increased risk of genital infection was noted across EMPA vs placebo (risk ratio: 2.59, 95% confidence interval: 1.80-3.71, P < .00001).
Conclusion: Our evidence supports the application of EMPA in treatment of patients with T2DM who are obesity or at risk of weight gain.
Figures






Similar articles
-
Empagliflozin for Type 2 Diabetes Mellitus: An Overview of Phase 3 Clinical Trials.Curr Diabetes Rev. 2017;13(4):405-423. doi: 10.2174/1573399812666160613113556. Curr Diabetes Rev. 2017. PMID: 27296042 Free PMC article. Review.
-
Efficacy and safety of empagliflozin in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials.Postgrad Med. 2017 Apr;129(3):382-392. doi: 10.1080/00325481.2017.1259544. Epub 2016 Nov 25. Postgrad Med. 2017. PMID: 27841714 Review.
-
Empagliflozin as Add-on Therapy to Pioglitazone With or Without Metformin in Patients With Type 2 Diabetes Mellitus.Clin Ther. 2015 Aug;37(8):1773-88.e1. doi: 10.1016/j.clinthera.2015.05.511. Epub 2015 Jun 29. Clin Ther. 2015. PMID: 26138864 Clinical Trial.
-
Pooled Safety and Tolerability Analysis of Empagliflozin in Patients with Type 2 Diabetes Mellitus.Adv Ther. 2020 Aug;37(8):3463-3484. doi: 10.1007/s12325-020-01329-7. Epub 2020 May 5. Adv Ther. 2020. PMID: 32372290 Free PMC article.
-
Safety and tolerability of empagliflozin in East Asian patients with type 2 diabetes: Pooled analysis of phase I-III clinical trials.J Diabetes Investig. 2019 Mar;10(2):418-428. doi: 10.1111/jdi.12910. Epub 2018 Sep 20. J Diabetes Investig. 2019. PMID: 30099847 Free PMC article.
Cited by
-
Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors.Chronic Dis Transl Med. 2020 Aug 15;6(4):239-245. doi: 10.1016/j.cdtm.2020.07.002. eCollection 2020 Dec. Chronic Dis Transl Med. 2020. PMID: 33336169 Free PMC article. Review.
-
Safety of sodium-glucose transporter 2 (SGLT-2) inhibitors in patients with type 2 diabetes: a meta-analysis of cohort studies.Front Pharmacol. 2023 Oct 13;14:1275060. doi: 10.3389/fphar.2023.1275060. eCollection 2023. Front Pharmacol. 2023. PMID: 37905204 Free PMC article.
-
Treatment of Type 2 Diabetes by Patient Profile in the Clinical Practice of Endocrinology in Spain: Delphi Study Results from the Think Twice Program.Diabetes Ther. 2019 Oct;10(5):1893-1907. doi: 10.1007/s13300-019-0671-x. Epub 2019 Jul 29. Diabetes Ther. 2019. PMID: 31359366 Free PMC article.
-
Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Transcription Regulation of AgRP and POMC Genes.Curr Issues Mol Biol. 2024 Jul 15;46(7):7505-7515. doi: 10.3390/cimb46070445. Curr Issues Mol Biol. 2024. PMID: 39057086 Free PMC article.
-
Urogenital infections in patients with diabetes mellitus: Beyond the conventional aspects.Int J Immunopathol Pharmacol. 2019 Jan-Dec;33:2058738419866582. doi: 10.1177/2058738419866582. Int J Immunopathol Pharmacol. 2019. PMID: 32031031 Free PMC article.
References
-
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14:5–14. - PubMed
-
- Lewin A, DeFronzo RA, Patel S, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care 2015;38:394–402. - PubMed
-
- Ross S, Thamer C, Cescutti J, et al. Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2015;17:699–702. - PubMed
-
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015;38:420–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

