Post-Translational Modifications and Diastolic Calcium Leak Associated to the Novel RyR2-D3638A Mutation Lead to CPVT in Patient-Specific hiPSC-Derived Cardiomyocytes
- PMID: 30413023
- PMCID: PMC6262462
- DOI: 10.3390/jcm7110423
Post-Translational Modifications and Diastolic Calcium Leak Associated to the Novel RyR2-D3638A Mutation Lead to CPVT in Patient-Specific hiPSC-Derived Cardiomyocytes
Abstract
Background: Sarcoplasmic reticulum Ca2+ leak and post-translational modifications under stress have been implicated in catecholaminergic polymorphic ventricular tachycardia (CPVT), a highly lethal inherited arrhythmogenic disorder. Human induced pluripotent stem cells (hiPSCs) offer a unique opportunity for disease modeling.
Objective: The aims were to obtain functional hiPSC-derived cardiomyocytes from a CPVT patient harboring a novel ryanodine receptor (RyR2) mutation and model the syndrome, drug responses and investigate the molecular mechanisms associated to the CPVT syndrome.
Methods: Patient-specific cardiomyocytes were generated from a young athletic female diagnosed with CPVT. The contractile, intracellular Ca2+ handling and electrophysiological properties as well as the RyR2 macromolecular remodeling were studied.
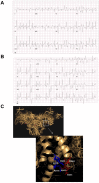
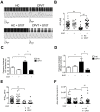
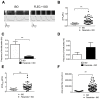
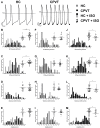
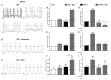
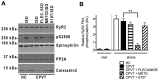
Results: Exercise stress electrocardiography revealed polymorphic ventricular tachycardia when treated with metoprolol and marked improvement with flecainide alone. We found abnormal stress-induced contractile and electrophysiological properties associated with sarcoplasmic reticulum Ca2+ leak in CPVT hiPSC-derived cardiomyocytes. We found inadequate response to metoprolol and a potent response of flecainide. Stabilizing RyR2 with a Rycal compound prevents those abnormalities specifically in CPVT hiPSC-derived cardiomyocytes. The RyR2-D3638A mutation is located in the conformational change inducing-central core domain and leads to RyR2 macromolecular remodeling including depletion of PP2A and Calstabin2.
Conclusion: We identified a novel RyR2-D3638A mutation causing 3D conformational defects and aberrant biophysical properties associated to RyR2 macromolecular complex post-translational remodeling. The molecular remodeling is for the first time revealed using patient-specific hiPSC-derived cardiomyocytes which may explain the CPVT proband's resistance. Our study promotes hiPSC-derived cardiomyocytes as a suitable model for disease modeling, testing new therapeutic compounds, personalized medicine and deciphering underlying molecular mechanisms.
Keywords: CPVT; calcium; flecainide; hiPSC-derived cardiomyocytes; post-translational modifications; ryanodine receptor; β-adrenergic receptor blockade.
Conflict of interest statement
A.R.M. is a board member and owns shares in ARMGO Pharma Inc., which is targeting RyR channels for therapeutic purposes.
Figures


References
-
- Liang P., Sallam K., Wu H., Li Y., Itzhaki I., Garg P., Zhang Y., Termglichan V., Lan F., Gu M., et al. Patient-Specific and Genome-Edited Induced Pluripotent Stem Cell–Derived Cardiomyocytes Elucidate Single-Cell Phenotype of Brugada Syndrome. J. Am. Coll. Cardiol. 2016;68:2086–2096. doi: 10.1016/j.jacc.2016.07.779. - DOI - PMC - PubMed
-
- Moreau A., Mercier A., Theriault O., Boutjdir M., Burger B., Keller D.I., Chahine M. Biophysical, Molecular, and Pharmacological Characterization of Voltage-Dependent Sodium Channels from Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Can. J. Cardiol. 2017;33:269–278. doi: 10.1016/j.cjca.2016.10.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

