Group II innate lymphoid cells and microvascular dysfunction from pulmonary titanium dioxide nanoparticle exposure
- PMID: 30413212
- PMCID: PMC6230229
- DOI: 10.1186/s12989-018-0280-2
Group II innate lymphoid cells and microvascular dysfunction from pulmonary titanium dioxide nanoparticle exposure
Abstract
Background: The cardiovascular effects of pulmonary exposure to engineered nanomaterials (ENM) are poorly understood, and the reproductive consequences are even less understood. Inflammation remains the most frequently explored mechanism of ENM toxicity. However, the key mediators and steps between lung exposure and uterine health remain to be fully defined. The purpose of this study was to determine the uterine inflammatory and vascular effects of pulmonary exposure to titanium dioxide nanoparticles (nano-TiO2). We hypothesized that pulmonary nano-TiO2 exposure initiates a Th2 inflammatory response mediated by Group II innate lymphoid cells (ILC2), which may be associated with an impairment in uterine microvascular reactivity.
Methods: Female, virgin, Sprague-Dawley rats (8-12 weeks) were exposed to 100 μg of nano-TiO2 via intratracheal instillation 24 h prior to microvascular assessments. Serial blood samples were obtained at 0, 1, 2 and 4 h post-exposure for multiplex cytokine analysis. ILC2 numbers in the lungs were determined. ILC2s were isolated and phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) levels were measured. Pressure myography was used to assess vascular reactivity of isolated radial arterioles.
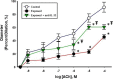
Results: Pulmonary nano-TiO2 exposure was associated with an increase in IL-1ß, 4, 5 and 13 and TNF- α 4 h post-exposure, indicative of an innate Th2 inflammatory response. ILC2 numbers were significantly increased in lungs from exposed animals (1.66 ± 0.19%) compared to controls (0.19 ± 0.22%). Phosphorylation of the transactivation domain (Ser-468) of NF-κB in isolated ILC2 and IL-33 in lung epithelial cells were significantly increased (126.8 ± 4.3% and 137 ± 11% of controls respectively) by nano-TiO2 exposure. Lastly, radial endothelium-dependent arteriolar reactivity was significantly impaired (27 ± 12%), while endothelium-independent dilation (7 ± 14%) and α-adrenergic sensitivity (8 ± 2%) were not altered compared to control levels. Treatment with an anti- IL-33 antibody (1 mg/kg) 30 min prior to nano-TiO2 exposure resulted in a significant improvement in endothelium-dependent dilation and a decreased level of IL-33 in both plasma and bronchoalveolar lavage fluid.
Conclusions: These results provide evidence that the uterine microvascular dysfunction that follows pulmonary ENM exposure may be initiated via activation of lung-resident ILC2 and subsequent systemic Th2-dependent inflammation.
Keywords: Engineered nanomaterials; Inflammation; Innate lymphoid cells; Microcirculation; Titanium dioxide nanoparticles.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
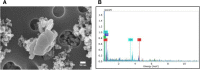

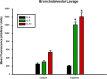
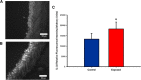




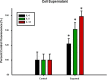
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

