Inhibitor clinical burden of disease: a comparative analysis of the CHESS data
- PMID: 30413215
- PMCID: PMC6230298
- DOI: 10.1186/s13023-018-0929-9
Inhibitor clinical burden of disease: a comparative analysis of the CHESS data
Abstract
Background: Patients with hemophilia and inhibitors generally face greater disease burden compared to patients without inhibitors. While raising awareness of relative burden may improve the standard of care for patients with inhibitors, comparative data are sparse. Analyzing data drawn from the Cost of Haemophilia across Europe - a Socioeconomic Survey (CHESS) study, the aim of this study was to compare the clinical burden of disease in patients with severe hemophilia with and without inhibitors. Hemophilia specialists (N = 139) across five European countries completed an online survey between January-April 2015, providing demographic, clinical and 12-month ambulatory/secondary care activity data for 1285 patients. Patients with hemophilia who currently presented with inhibitors and those who never had inhibitors were matched on baseline characteristics via propensity score matching. Outcomes were compared between the two cohorts using a paired t-test or Wilcoxon signed-rank or McNemar's test.
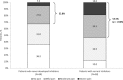
Results: The proportion of patients who currently presented with inhibitors was 4.5% (58/1285). Compared to PS-matched patients without inhibitors, patients with inhibitors experienced more than twice the mean annual number of bleeds (mean ± standard deviation, 8.29 ± 9.18 vs 3.72 ± 3.95; p < .0001) and joint bleeds (2.17 ± 1.90 vs 0.98 ± 1.15; p < .0001), and required more hemophilia-related (mean ± standard deviation, 1.79 ± 1.83 vs 0.64 ± 1.13) and bleed-related hospitalizations (1.86 ± 1.88 vs 0.81 ± 1.26), hemophilia-related consultations (9.30 ± 4.99 vs 6.77 ± 4.47), and outpatient visits (22.09 ± 17.77 vs 11.48 ± 16.00) (all, p < .001). More than one-half (53.5%) experienced moderate/severe pain necessitating medication compared to one-third (32.8%) of patients without inhibitors (p = .01).
Conclusions: Patients with hemophilia and inhibitors exhibited greater clinical burden and higher resource utilization compared to their peers without inhibitors. Strategies for improving the standard of care may alleviate burden in this population.
Keywords: Disease burden; Hemophilia; Inhibitors.
Conflict of interest statement
Ethics approval and consent to participate
The CHESS study received ethics approval from the University of Chester Ethics Committee. All patients or their legal representatives provided signed informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
AO and ML are employees and shareholders of Shire. SW was an employee of HCD at the time the study was completed. TLK was an employee of Shire at the time the study was completed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Clinical, humanistic, and economic burden of severe hemophilia B in the United States: Results from the CHESS US and CHESS US+ population surveys.Orphanet J Rare Dis. 2021 Mar 20;16(1):143. doi: 10.1186/s13023-021-01774-9. Orphanet J Rare Dis. 2021. PMID: 33743752 Free PMC article.
-
Recombinant factor VIIa (eptacog alfa): a pharmacoeconomic review of its use in haemophilia in patients with inhibitors to clotting factors VIII or IX.Pharmacoeconomics. 2007;25(12):1007-29. doi: 10.2165/00019053-200725120-00004. Pharmacoeconomics. 2007. PMID: 18047387 Review.
-
The cost of severe haemophilia in Europe: the CHESS study.Orphanet J Rare Dis. 2017 May 31;12(1):106. doi: 10.1186/s13023-017-0660-y. Orphanet J Rare Dis. 2017. PMID: 28569181 Free PMC article.
-
Clinical, humanistic, and economic burden of severe haemophilia B in adults receiving factor IX prophylaxis: findings from the CHESS II real-world burden of illness study in Europe.Orphanet J Rare Dis. 2021 Dec 20;16(1):521. doi: 10.1186/s13023-021-02152-1. Orphanet J Rare Dis. 2021. PMID: 34930388 Free PMC article.
-
FEIBA versus NovoSeven in hemophilia patients with inhibitors.Semin Thromb Hemost. 2013 Oct;39(7):772-8. doi: 10.1055/s-0033-1354425. Epub 2013 Sep 8. Semin Thromb Hemost. 2013. PMID: 24014071 Review.
Cited by
-
Reduced Volume and Faster Infusion Rate of Activated Prothrombin Complex Concentrate: A Phase 3b/4 Trial in Adults with Hemophilia A with Inhibitors.TH Open. 2024 Jul 8;8(3):e273-e282. doi: 10.1055/s-0044-1787781. eCollection 2024 Jul. TH Open. 2024. PMID: 38983688 Free PMC article.
-
Effectiveness of Prophylactic Coagulation Factor Replacement Therapy in Patients with Severe Hemophilia A in Taiwan - A Population-Based Study.Clin Epidemiol. 2022 Dec 13;14:1501-1510. doi: 10.2147/CLEP.S391753. eCollection 2022. Clin Epidemiol. 2022. PMID: 36536898 Free PMC article.
-
Defining the impact of immune tolerance induction on clinically relevant outcomes in a US cohort of severe hemophilia A.Blood Adv. 2024 Mar 12;8(5):1190-1199. doi: 10.1182/bloodadvances.2023011974. Blood Adv. 2024. PMID: 38163316 Free PMC article.
-
Emicizumab prophylaxis in infants with hemophilia A (HAVEN 7): primary analysis of a phase 3b open-label trial.Blood. 2024 Apr 4;143(14):1355-1364. doi: 10.1182/blood.2023021832. Blood. 2024. PMID: 38127586 Free PMC article. Clinical Trial.
-
Gut dysbiosis modulates the immune response to factor VIII in murine hemophilia A.Blood Adv. 2020 Jun 23;4(12):2644-2655. doi: 10.1182/bloodadvances.2019001144. Blood Adv. 2020. PMID: 32556285 Free PMC article.
References
-
- World Federation of Hemophilia. Report on the Annual Global Survey 2015. October 2016. Available at: http://www1.wfh.org/publication/files/pdf-1669.pdf. Accessed October 19, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

