Manganese Oxide Biomineralization Provides Protection against Nitrite Toxicity in a Cell-Density-Dependent Manner
- PMID: 30413475
- PMCID: PMC6328764
- DOI: 10.1128/AEM.02129-18
Manganese Oxide Biomineralization Provides Protection against Nitrite Toxicity in a Cell-Density-Dependent Manner
Abstract
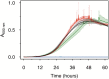
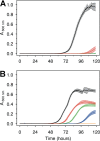
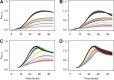
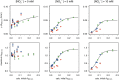
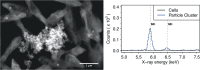
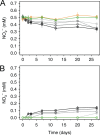
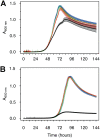
Manganese biomineralization is a widespread process among bacteria and fungi. To date, there is no conclusive experimental evidence for how and if this process impacts microbial fitness in the environment. Here, we show how a model organism for manganese oxidation is growth inhibited by nitrite, and that this inhibition is mitigated in the presence of manganese. We show that such manganese-mediated mitigation of nitrite inhibition is dependent on the culture inoculum size, and that manganese oxide (MnOX) forms granular precipitates in the culture, rather than sheaths around individual cells. We provide evidence that MnOX protection involves both its ability to catalyze nitrite oxidation into (nontoxic) nitrate under physiological conditions and its potential role in influencing processes involving reactive oxygen species (ROS). Taken together, these results demonstrate improved microbial fitness through MnOX deposition in an ecological setting, i.e., mitigation of nitrite toxicity, and point to a key role of MnOX in handling stresses arising from ROS.IMPORTANCE We present here a direct fitness benefit (i.e., growth advantage) for manganese oxide biomineralization activity in Roseobacter sp. strain AzwK-3b, a model organism used to study this process. We find that strain AzwK-3b in a laboratory culture experiment is growth inhibited by nitrite in manganese-free cultures, while the inhibition is considerably relieved by manganese supplementation and manganese oxide (MnOX) formation. We show that biogenic MnOX interacts directly with nitrite and possibly with reactive oxygen species and find that its beneficial effects are established through formation of dispersed MnOX granules in a manner dependent on the population size. These experiments raise the possibility that manganese biomineralization could confer protection against nitrite toxicity to a population of cells. They open up new avenues of interrogating this process in other species and provide possible routes to their biotechnological applications, including in metal recovery, biomaterials production, and synthetic community engineering.
Keywords: Roseobacter; biomineralization; metal recovery; microbial ecology; reactive oxygen species; respiration.
Copyright © 2019 Zerfaß et al.
Figures
References
-
- Hansel CM. 2017. Manganese in marine microbiology, p 37–83. In Poole RK. (ed), Advances in microbial physiology: microbiology of metal ions. Academic Press, Oxford, UK. - PubMed
-
- Nealson KH. 2006. The manganese-oxidizing bacteria. Prokaryotes 5:222–231.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

