Teaching an old dog new tricks: next-generation CAR T cells
- PMID: 30413825
- PMCID: PMC6325111
- DOI: 10.1038/s41416-018-0325-1
Teaching an old dog new tricks: next-generation CAR T cells
Abstract
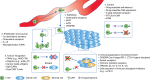
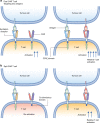
Adoptive T cell therapy (ACT) refers to the therapeutic use of T cells. T cells genetically engineered to express chimeric antigen receptors (CAR) constitute the most clinically advanced form of ACT approved to date for the treatment of CD19-positive leukaemias and lymphomas. CARs are synthetic receptors that are able to confer antigen-binding and activating functions on T cells with the aim of therapeutically targeting cancer cells. Several factors are essential for CAR T cell therapy to be effective, such as recruitment, activation, expansion and persistence of bioengineered T cells at the tumour site. Despite the advances made in CAR T cell therapy, however, most tumour entities still escape immune detection and elimination. A number of strategies counteracting these problems will need to be addressed in order to render T cell therapy effective in more situations than currently possible. Non-haematological tumours are also the subject of active investigation, but ACT has so far shown only marginal success rates in these cases. New approaches are needed to enhance the ability of ACT to target solid tumours without increasing toxicity, by improving recognition, infiltration, and persistence within tumours, as well as an enhanced resistance to the suppressive tumour microenvironment.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

