Mebendazole and radiation in combination increase survival through anticancer mechanisms in an intracranial rodent model of malignant meningioma
- PMID: 30414098
- PMCID: PMC6387633
- DOI: 10.1007/s11060-018-03009-7
Mebendazole and radiation in combination increase survival through anticancer mechanisms in an intracranial rodent model of malignant meningioma
Abstract
Purpose: Meningiomas are a frequent tumor of the central nervous system. Although mostly benign, approximately 5% present as atypical or malignant tumors. Treatments for atypical meningiomas include gross total resection and radiotherapy, but about 33% of patients have recurrent tumors, sometimes as a higher grade. Recently, the brain penetrant anthelmintic drug, mebendazole, has shown promise as an anticancer agent in rodent models of glioblastoma and medulloblastoma.
Methods: The half maximal inhibitory concentration (IC50) effect on colony formation, cell proliferation, and caspase-3/7 markers of apoptosis of mebendazole with and without radiation was measured in vitro. Mice intracranially implanted with KT21MG1 human meningioma were administered mebendazole alone or in combination with radiation. Survival benefit was evaluated, while tumors were investigated by immunohistochemical staining for apoptosis, cell proliferation, and vascular density.
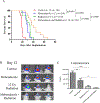
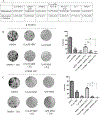
Results: In vitro experiments on meningioma cell lines showed the IC50 for mebendazole in the range of 0.26-0.42 µM. Mebendazole alone induced cytotoxicity, however the combination had a greater reduction in colony formation and resulted in higher levels of cleaved caspase-3. The in vivo study showed both, mebendazole alone and the combination, to have a survival benefit with an increase in apoptosis, and decreases in tumor cell and vascular proliferation.
Conclusion: These preclinical findings indicate that mebendazole alone or in combination with radiation can be considered for the treatment of malignant meningioma. The mechanism of action for this combination may include an increase in apoptosis, a reduction in proliferation and angiogenesis, or a combination of these effects.
Keywords: Brain; Mebendazole; Meningioma; Preclinical; Radiation.
Conflict of interest statement
Conflict of Interest
GJR has a financial interest in Benizole Therapeutics, PBC and in mebendazole related intellectual property managed by Johns Hopkins University.
Figures



Similar articles
-
Nelfinavir potentiation of imatinib cytotoxicity in meningioma cells via survivin inhibition.Neurosurg Focus. 2007;23(4):E9. doi: 10.3171/FOC-07/10/E9. Neurosurg Focus. 2007. PMID: 17961046
-
Calcium channel antagonists augment hydroxyurea- and ru486-induced inhibition of meningioma growth in vivo and in vitro.Neurosurgery. 2006 Nov;59(5):1109-20; discussion 1120-1. doi: 10.1227/01.NEU.0000245597.46581.FB. Neurosurgery. 2006. PMID: 17143245
-
The integrin inhibitor cilengitide affects meningioma cell motility and invasion.Clin Cancer Res. 2013 Oct 1;19(19):5402-12. doi: 10.1158/1078-0432.CCR-12-0299. Epub 2013 Aug 15. Clin Cancer Res. 2013. PMID: 23948974
-
Medical treatment of recurrent meningiomas.Expert Rev Neurother. 2011 Oct;11(10):1425-32. doi: 10.1586/ern.11.38. Expert Rev Neurother. 2011. PMID: 21955199 Review.
-
Radiotherapy for atypical or malignant intracranial meningioma.Int J Radiat Oncol Biol Phys. 1996 Mar 1;34(4):817-22. doi: 10.1016/0360-3016(95)02166-3. Int J Radiat Oncol Biol Phys. 1996. PMID: 8598358 Review.
Cited by
-
Repurposing of Benzimidazole Anthelmintic Drugs as Cancer Therapeutics.Cancers (Basel). 2022 Sep 22;14(19):4601. doi: 10.3390/cancers14194601. Cancers (Basel). 2022. PMID: 36230527 Free PMC article. Review.
-
Mebendazole effectively overcomes imatinib resistance by dual-targeting BCR/ABL oncoprotein and β-tubulin in chronic myeloid leukemia cells.Korean J Physiol Pharmacol. 2025 Jan 1;29(1):67-81. doi: 10.4196/kjpp.24.176. Epub 2024 Nov 14. Korean J Physiol Pharmacol. 2025. PMID: 39539176 Free PMC article.
-
Newly established patient-derived organoid model of intracranial meningioma.Neuro Oncol. 2021 Nov 2;23(11):1936-1948. doi: 10.1093/neuonc/noab155. Neuro Oncol. 2021. PMID: 34214169 Free PMC article.
-
Anticancer Effect of Benzimidazole Derivatives, Especially Mebendazole, on Triple-Negative Breast Cancer (TNBC) and Radiotherapy-Resistant TNBC In Vivo and In Vitro.Molecules. 2021 Aug 24;26(17):5118. doi: 10.3390/molecules26175118. Molecules. 2021. PMID: 34500557 Free PMC article.
-
Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: results of a phase 1 clinical trial.Neurooncol Adv. 2020 Nov 12;3(1):vdaa154. doi: 10.1093/noajnl/vdaa154. eCollection 2021 Jan-Dec. Neurooncol Adv. 2020. PMID: 33506200 Free PMC article.
References
-
- Kessler RA, et al., Metastatic Atypical and Anaplastic Meningioma: A Case Series and Review of the Literature. World Neurosurg, 2017. 101: p. 47–56. - PubMed
-
- Moazzam AA, Wagle N, and Zada G, Recent developments in chemotherapy for meningiomas: a review. Neurosurg Focus, 2013. 35(6): p. E18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

