Reduction in albuminuria with dapagliflozin cannot be predicted by baseline clinical characteristics or changes in most other risk markers
- PMID: 30414240
- PMCID: PMC6590413
- DOI: 10.1111/dom.13579
Reduction in albuminuria with dapagliflozin cannot be predicted by baseline clinical characteristics or changes in most other risk markers
Abstract
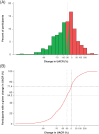
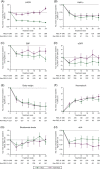
The sodium glucose co-transporter-2 inhibitor dapagliflozin has been shown to decrease urinary albumin-to-creatinine ratio (UACR). This effect, however, varies among individual patients. In this study, we assessed the baseline characteristics and concurrent changes in other cardiovascular risk markers that might be associated with UACR response to dapagliflozin. A pooled analysis of 11 phase 3 randomized, controlled clinical trials was performed. UACR change from baseline after 24 weeks treatment with dapagliflozin 10 mg/d in 531 patients with type 2 diabetes and UACR ≥30 mg/g at baseline was determined. UACR response was defined as >30% reduction from baseline at 24 weeks, whereas UACR non-response was defined as ≤30% reduction at 24 weeks. A total of 288 (54%) patients were classified as responders and 243 (46%) as non-responders. At 24 weeks, the UACR-adjusted mean change from baseline was -71.2% and 25.9% in responders and non-responders, respectively. Baseline characteristics were similar between both groups. Changes in HbA1c and body weight were comparable across groups. Responders showed a numerically larger reduction in estimated glomerular filtration rate and systolic blood pressure versus non-responders. UACR reduction to dapagliflozin is an individual characteristic that cannot be predicted by baseline clinical features or changes in metabolic variables. Whether UACR response would improve long-term renal and cardiovascular outcomes remains to be determined.
Keywords: albuminuria; dapagliflozin; diabetes; hypertension; sodium glucose co-transporter-2.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
P.S., C.D.S., and B.V.S. designed the study. H.J.L.H. and P.S. wrote the first draft of this manuscript. V.C. performed all statistical analyses. All authors contributed to interpretation and critical revisions of the manuscript. All authors approved the submission for publication. H.J.L.H. and P.S. take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Figures
References
-
- Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375:2223‐2233. - PubMed
-
- Weber M, Mansfield T, Cain V, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211‐220. - PubMed
-
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. - PubMed
-
- Petrykiv SI, Laverman GD, de Zeeuw D, Heerspink HJL. The albuminuria‐lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab. 2017;19:1363‐1370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

