Regulating a key mitotic regulator, polo-like kinase 1 (PLK1)
- PMID: 30414309
- PMCID: PMC7113694
- DOI: 10.1002/cm.21504
Regulating a key mitotic regulator, polo-like kinase 1 (PLK1)
Abstract
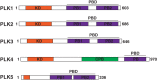
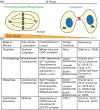
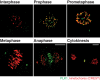
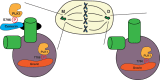
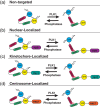
During cell division, duplicated genetic material is separated into two distinct daughter cells. This process is essential for initial tissue formation during development and to maintain tissue integrity throughout an organism's lifetime. To ensure the efficacy and efficiency of this process, the cell employs a variety of regulatory and signaling proteins that function as mitotic regulators and checkpoint proteins. One vital mitotic regulator is polo-like kinase 1 (PLK1), a highly conserved member of the polo-like kinase family. Unique from its paralogues, it functions specifically during mitosis as a regulator of cell division. PLK1 is spatially and temporally enriched at three distinct subcellular locales; the mitotic centrosomes, kinetochores, and the cytokinetic midbody. These localization patterns allow PLK1 to phosphorylate specific downstream targets to regulate mitosis. In this review, we will explore how polo-like kinases were originally discovered and diverged into the five paralogues (PLK1-5) in mammals. We will then focus specifically on the most conserved, PLK1, where we will discuss what is known about how its activity is modulated, its role during the cell cycle, and new, innovative tools that have been developed to examine its function and interactions in cells.
Keywords: FRET; biosensor; cell division; centrosome; chemical genetics; kinetochore; midbody; mitosis; polo-like kinase 1; scaffolds.
© 2018 The Authors. Cytoskeleton published by Wiley Periodicals, Inc.
Figures

References
-
- Awad, M. M. , Chu, Q. S. C. , Gandhi, L. , Stephenson, J. J. , Govindan, R. , Bradford, D. S. , … Socinski, M. A. (2017). An open‐label, phase II study of the polo‐like kinase‐1 (Plk‐1) inhibitor, BI 2536, in patients with relapsed small cell lung cancer (SCLC). Lung Cancer, 104, 126–130. 10.1016/j.lungcan.2016.12.019 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

