Deletion of nicotinic acetylcholine receptor alpha9 in mice resulted in altered bone structure
- PMID: 30414510
- PMCID: PMC6492625
- DOI: 10.1016/j.bone.2018.11.003
Deletion of nicotinic acetylcholine receptor alpha9 in mice resulted in altered bone structure
Abstract
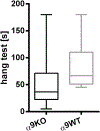
Alterations in bone strength and structure were found in knockout (KO) mouse strains with deletion of several acetylcholine receptors. Interestingly, the expression of the nicotinic acetylcholine receptors (nAChR) subunit α10 was down-regulated in osteogenic differentiated mesenchymal stem cells of patients with osteoporosis whereas the expression of subunit α9 was not altered. Since nAChR subunits α9 and α10 are often combined in a functional receptor, we analyzed here the bone of adult female KO mice with single deletion of either nAChR alpha9 (α9KO) or alpha10 (α10KO). Biomechanical testing showed a significant decrease of bending stiffness and maximal breaking force in α9KO compared to their corresponding wild type mice. Furthermore, an increase in trabecular pattern factor (Tb.Pf) and structure model index (SMI) was detected by μCT in α9KO indicating reduced bone mass. On the mRNA level a decrease of Collagen 1α1 and Connexin-43 was measured by real-time RT-PCR in α9KO while no alteration of osteoclast markers was detected in either mouse strain. Using electron microcopy we observed an increase in the number of osteocytes that showed signs of degeneration and cell death in the α9KO compared to their wild type mice, while α10KO showed no differences. In conclusion, we demonstrate alterations in bone strength, structure and bio-marker expression in α9KO mice which imply the induction of osteocyte degeneration. Thus, our data suggest that nAChR containing the α9 subunit might be involved in the homeostasis of osteocytes and therefore in bone mass regulation.
Keywords: Bending stiffness; Collagen 1α1; Connexin-43; Micro-CT; Non-neuronal cholinergic system; nAChR α10.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures
All authors state that they have no conflict of interest.
Figures





References
-
- Lips KS, Kneffel M, Willscheid F, Mathies FM, Kampschulte M, Hartmann S, Panzer I, Durselen L, Heiss C, Kauschke V, Altered ultrastructure, density and cathepsin K expression in bone of female muscarinic acetylcholine receptor M3 knockout mice, Int. Immunopharmacol 29 (2015) 201–207. - PubMed
-
- Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M, Tzartos SJ, Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity, FEBS J. 274 (2007) 3799–3845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

