Efficacy of continuous in-wound infusion of levobupivacaine and ketorolac for post-caesarean section analgesia: a prospective, randomised, double-blind, placebo-controlled trial
- PMID: 30414609
- PMCID: PMC6234771
- DOI: 10.1186/s12871-018-0609-2
Efficacy of continuous in-wound infusion of levobupivacaine and ketorolac for post-caesarean section analgesia: a prospective, randomised, double-blind, placebo-controlled trial
Abstract
Background: In-wound catheters for infusion of local anaesthetic for post-caesarean section analgesia are well tolerated in parturients. Few studies have examined continuous in-wound infusion of a combination of local anaesthetic and non-steroidal anti-inflammatory drug for post-caesarean section analgesia. This single centre study evaluated post-operative analgesic efficacy and piritramide-sparing effects of continuous in-wound infusion of either local anaesthetic or non-steroidal anti-inflammatory agent, or the combination of both, versus saline placebo, when added to systemic analgesia with paracetamol.
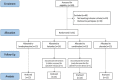
Methods: After National Ethical Board approval, 59 pregnant women scheduled for non-emergency caesarean section were included in this prospective, randomised, double-blind, placebo-controlled study. The parturients received spinal anaesthesia with levobupivacaine and fentanyl. Post-operative analgesia to 48 h included paracetamol 1000 mg intravenously every 6 h, with the studied agents as in-wound infusions. Rescue analgesia with piritramide was available as needed, titrated to 2 mg intravenously. Four groups were compared, using a subcutaneous multi-holed catheter connected to an elastomeric pump running at 5 mL/h over 48 h. The different in-wound infusions were: levobupivacaine 0.25% alone; ketorolac tromethamine 0.08% alone; levobupivacaine 0.25% plus ketorolac tromethamine 0.08%; or saline placebo. The primary outcome was total rescue piritramide used at 24 h and 48 h post-operatively, under maintained optimal post-caesarean section analgesia.
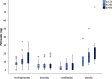
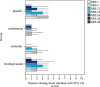
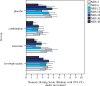
Results: Compared to placebo in-wound infusions, ketorolac alone and levobupivacaine plus ketorolac in-wound infusions both significantly reduced post-operative piritramide consumption at 24 h (p = 0.003; p < 0.001, respectively) and 48 h (p = 0.001; p < 0.001). Compared to levobupivacaine, levobupivacaine plus ketorolac significantly reduced post-operative piritramide consumption at 24 h (p = 0.015) and 48 h (p = 0.021). For levobupivacaine versus ketorolac, no significant differences were seen for post-operative piritramide consumption at 24 h and 48 h (p = 0.141; p = 0.054).
Conclusion: Continuous in-wound infusion with levobupivacaine plus ketorolac provides greater opioid-sparing effects than continuous in-wound infusion with levobupivacaine alone.
Trial registration: German Clinical Trials Register: retrospectively registered on 30 July, 2014, DRKS 00006559 .
Keywords: Analgesia; Caesarean section; In-wound infusion; Ketorolac; Levobupivacaine.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the National Medical Ethics Committee (Republic of Slovenia National Medical Ethics Committee, Number 169/07/11) on July 12, 2011. All of the participants gave their written, informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Ropivacaine and Ketorolac Wound Infusion for Post-Cesarean Delivery Analgesia: A Randomized Controlled Trial.Obstet Gynecol. 2020 Feb;135(2):427-435. doi: 10.1097/AOG.0000000000003601. Obstet Gynecol. 2020. PMID: 31923061 Clinical Trial.
-
Postoperative analgesia after cesarean section by continued administration of levobupivacaine with the On-Q Painbuster system over the fascia vs ketorolac + morphine i.v.Clin Exp Obstet Gynecol. 2006;33(4):223-5. Clin Exp Obstet Gynecol. 2006. PMID: 17211970 Clinical Trial.
-
Posteromedial quadratus lumborum block versus wound infiltration after caesarean section: A randomised, double-blind, controlled study.Eur J Anaesthesiol. 2021 Aug 1;38(Suppl 2):S138-S144. doi: 10.1097/EJA.0000000000001531. Eur J Anaesthesiol. 2021. PMID: 33988528 Clinical Trial.
-
Transversus abdominis plane block versus local anaesthetic wound infiltration for analgesia after caesarean section: A systematic review and meta-analysis with trial sequential analysis.Eur J Anaesthesiol. 2022 Mar 1;39(3):244-251. doi: 10.1097/EJA.0000000000001552. Eur J Anaesthesiol. 2022. PMID: 34091477
-
Caesarean section wound infiltration with local anaesthesia for postoperative pain relief - any benefit?S Afr Med J. 2010 May 4;100(5):313-9. doi: 10.7196/samj.3716. S Afr Med J. 2010. PMID: 20460027 Review.
Cited by
-
The effect of a local anesthetic cocktail in a serratus anterior plane and PECS 1 block for implant-based breast reconstruction.JPRAS Open. 2024 May 23;41:116-127. doi: 10.1016/j.jpra.2024.04.008. eCollection 2024 Sep. JPRAS Open. 2024. PMID: 38984322 Free PMC article.
-
Comparison of analgesic modalities after cesarean section: a network meta-analysis and systematic review.Int J Surg. 2025 May 1;111(5):3599-3612. doi: 10.1097/JS9.0000000000002352. Int J Surg. 2025. PMID: 40146253 Free PMC article.
-
Continuous Wound Infiltration of Local Anesthetics in Postoperative Pain Management: Safety, Efficacy and Current Perspectives.J Pain Res. 2020 Jan 31;13:285-294. doi: 10.2147/JPR.S211234. eCollection 2020. J Pain Res. 2020. PMID: 32099452 Free PMC article. Review.
-
The Efficacy and Safety of Local Anesthetic Techniques for Postoperative Analgesia After Cesarean Section: A Bayesian Network Meta-Analysis of Randomized Controlled Trials.J Pain Res. 2021 Jun 2;14:1559-1572. doi: 10.2147/JPR.S313972. eCollection 2021. J Pain Res. 2021. PMID: 34103981 Free PMC article.
-
Stability and compatibility of admixtures containing bupivacaine hydrochloride and ketorolac tromethamine for parenteral use.Eur J Hosp Pharm. 2023 Mar;30(e1):e48-e54. doi: 10.1136/ejhpharm-2021-003003. Epub 2021 Oct 18. Eur J Hosp Pharm. 2023. PMID: 34663584 Free PMC article.
References
-
- Gibbons L, Belizan JM, Lauer JA, Betran AP, Merialdi M, Althabe F. The Global Numbers and Costs of Additionally Needed and Unnecessary Caesarean Sections Performed per Year: Overuse as a Barrier to Universal Coverage. World Health Report (2010), Background Paper 30. Geneva: World Health Organization; 2010.
-
- OECD . Caesarean sections, in health at a glance 2015: OECD indicators, OECD publishing. Paris: DOI; 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

