A Preclinical Model for Studying Herpes Simplex Virus Infection
- PMID: 30414908
- PMCID: PMC7100788
- DOI: 10.1016/j.jid.2018.08.034
A Preclinical Model for Studying Herpes Simplex Virus Infection
Abstract
Herpes simplex virus (HSV) infections can cause considerable morbidity. Currently, nucleoside analogues such as acyclovir are widely used for treatment. However, HSV infections resistant to these drugs are a clinical problem among immunocompromised patients. To provide more efficient therapy and to counteract resistance, a different class of antiviral compounds has been developed. Pritelivir, a helicase primase inhibitor, represents a promising candidate for improved therapy. Here, we established an HSV-1 infection model on microneedle-pretreated human skin ex vivo. We identified HSV-1-specific histological changes (e.g., cytopathic effects, multinucleated giant cells), down-regulation of nectin-1, nuclear translocation of NF-κB (p65), interferon regulatory factor 3 (IRF3), and signaling of the IFN-inducible protein MxA. Accordingly, this model was used to test the potency of pritelivir compared with the standard drug acyclovir. We discovered that both drugs had a comparable efficacy for inhibiting HSV-1 replication, suggesting that pritelivir could be an alternative therapeutic agent for patients infected with acyclovir-resistant strains. To our knowledge, we present a previously unreported ex vivo HSV-1 infection model with abdominal human skin to test antiviral drugs, thus bridging the gap between in vitro and in vivo drug screening and providing a valuable preclinical platform for HSV research.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors state on conflict of interest.
Figures
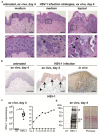
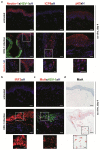
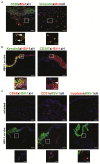

Comment in
-
Human Skin Explants Recapitulate Key Features of HSV-1 Infections.J Invest Dermatol. 2019 Mar;139(3):519-521. doi: 10.1016/j.jid.2018.09.015. J Invest Dermatol. 2019. PMID: 30797319
Similar articles
-
Pharmacokinetics-pharmacodynamics of the helicase-primase inhibitor pritelivir following treatment of wild-type or pritelivir-resistant virus infection in a murine herpes simplex virus 1 infection model.Antimicrob Agents Chemother. 2014 Jul;58(7):3843-52. doi: 10.1128/AAC.02641-14. Epub 2014 Apr 21. Antimicrob Agents Chemother. 2014. PMID: 24752278 Free PMC article.
-
Efficacy of pritelivir and acyclovir in the treatment of herpes simplex virus infections in a mouse model of herpes simplex encephalitis.Antiviral Res. 2018 Jan;149:1-6. doi: 10.1016/j.antiviral.2017.11.002. Epub 2017 Nov 4. Antiviral Res. 2018. PMID: 29113740 Free PMC article.
-
Helicase primase inhibitors (HPIs) are efficacious for therapy of human herpes simplex virus (HSV) disease in an infection mouse model.Antiviral Res. 2021 Nov;195:105190. doi: 10.1016/j.antiviral.2021.105190. Epub 2021 Oct 16. Antiviral Res. 2021. PMID: 34666109
-
HSV antivirals - current and future treatment options.Curr Opin Virol. 2016 Jun;18:9-13. doi: 10.1016/j.coviro.2016.01.013. Epub 2016 Feb 19. Curr Opin Virol. 2016. PMID: 26897058 Review.
-
Herpes simplex virus resistance to acyclovir: clinical relevance.Infect Agents Dis. 1995 Sep;4(3):115-24. Infect Agents Dis. 1995. PMID: 8548189 Review.
Cited by
-
Cytosolic DNA sensors activation of human astrocytes inhibits herpes simplex virus through IRF1 induction.Front Cell Infect Microbiol. 2024 May 14;14:1383811. doi: 10.3389/fcimb.2024.1383811. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38808062 Free PMC article.
-
Herpes simplex virus assembly and spread in murine skin after infection from the outside.J Virol. 2025 Mar 18;99(3):e0163824. doi: 10.1128/jvi.01638-24. Epub 2025 Feb 13. J Virol. 2025. PMID: 39945537 Free PMC article.
-
Engineering a Model to Study Viral Infections: Bioprinting, Microfluidics, and Organoids to Defeat Coronavirus Disease 2019 (COVID-19).Int J Bioprint. 2020 Aug 28;6(4):302. doi: 10.18063/ijb.v6i4.302. eCollection 2020. Int J Bioprint. 2020. PMID: 33089000 Free PMC article. Review.
-
The Extracellular Matrix in Skin Inflammation and Infection.Front Cell Dev Biol. 2021 Jul 6;9:682414. doi: 10.3389/fcell.2021.682414. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34295891 Free PMC article. Review.
-
3D Approaches to Culturing Bovine Skin: Explant Culture versus Organotypic Skin Model.Cells Tissues Organs. 2024;213(5):424-438. doi: 10.1159/000538438. Epub 2024 Mar 21. Cells Tissues Organs. 2024. PMID: 38508156 Free PMC article.
References
-
- Amici C, Rossi A, Costanzo A, Ciafrè S, Marinari B, Balsamo M, et al. Herpes simplex virus disrupts NF-κB regulation by blocking its recruitment on the IκBα promoter and directing the factor on viral genes. J Biol Chem. 2006;281:7110–7. - PubMed
-
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

