Dynamic control of hydrogel crosslinking via sortase-mediated reversible transpeptidation
- PMID: 30415064
- PMCID: PMC6697659
- DOI: 10.1016/j.actbio.2018.11.011
Dynamic control of hydrogel crosslinking via sortase-mediated reversible transpeptidation
Abstract
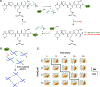
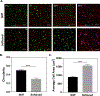
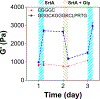
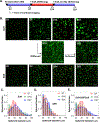
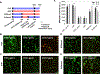
Cell-laden hydrogels whose crosslinking density can be dynamically and reversibly tuned are highly sought-after for studying pathophysiological cellular fate processes, including embryogenesis, fibrosis, and tumorigenesis. Special efforts have focused on controlling network crosslinking in poly(ethylene glycol) (PEG) based hydrogels to evaluate the impact of matrix mechanics on cell proliferation, morphogenesis, and differentiation. In this study, we sought to design dynamic PEG-peptide hydrogels that permit cyclic/reversible stiffening and softening. This was achieved by utilizing reversible enzymatic reactions that afford specificity, biorthogonality, and predictable reaction kinetics. To that end, we prepared PEG-peptide conjugates to enable sortase A (SrtA) induced tunable hydrogel crosslinking independent of macromer contents. Uniquely, these hydrogels can be completely degraded by the same enzymatic reactions and the degradation rate can be tuned from hours to days. We further synthesized SrtA-sensitive peptide linker (i.e., KCLPRTGCK) for crosslinking with 8-arm PEG-norbornene (PEG8NB) via thiol-norbornene photocrosslinking. These hydrogels afford diverse softening paradigms through control of network structures during crosslinking or by adjusting enzymatic parameters during on-demand softening. Importantly, user-controlled hydrogel softening promoted spreading of human mesenchymal stem cells (hMSCs) in 3D. Finally, we designed a bis-cysteine-bearing linear peptide flanked with SrtA substrates at the peptide's N- and C-termini (i.e., NH2-GGGCKGGGKCLPRTG-CONH2) to enable cyclic/reversible hydrogel stiffening/softening. We show that matrix stiffening and softening play a crucial role in growth and chemoresistance in pancreatic cancer cells. These results represent the first dynamic hydrogel platform that affords cyclic gel stiffening/softening based on reversible enzymatic reactions. More importantly, the chemical motifs that affords such reversible crosslinking were built-in on the linear peptide crosslinker without any post-synthesis modification. STATEMENT OF SIGNIFICANCE: Cell-laden 'dynamic' hydrogels are typically designed to enable externally stimulated stiffening or softening of the hydrogel network. However, no enzymatic reaction has been used to reversibly control matrix crosslinking. The application of SrtA-mediated transpeptidation in crosslinking and post-gelation modification of biomimetic hydrogels is innovative because of the specificity of the reaction and reversible tunability of crosslinking kinetics. While SrtA has been previously used to crosslink and fully degrade hydrogels, matrix softening and reversible stiffening of cell-laden hydrogels has not been reported. By designing simple peptide substrates, this unique enzymatic reaction can be employed to form a primary network, to gradually soften hydrogels, or to reversibly stiffen hydrogels. As a result, this dynamic hydrogel platform can be used to answer important matrix-related biological questions that are otherwise difficult to address.
Keywords: Cancer; Dynamic hydrogels; Extracellular matrix; Sortase A; Tissue stiffening.
Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells.Acta Biomater. 2017 Jan 15;48:258-269. doi: 10.1016/j.actbio.2016.10.027. Epub 2016 Oct 18. Acta Biomater. 2017. PMID: 27769941 Free PMC article.
-
Biomimetic stiffening of cell-laden hydrogels via sequential thiol-ene and hydrazone click reactions.Acta Biomater. 2021 Aug;130:161-171. doi: 10.1016/j.actbio.2021.05.054. Epub 2021 Jun 1. Acta Biomater. 2021. PMID: 34087443 Free PMC article.
-
Sortase A as a cross-linking enzyme in tissue engineering.Acta Biomater. 2018 Sep 1;77:182-190. doi: 10.1016/j.actbio.2018.07.020. Epub 2018 Jul 10. Acta Biomater. 2018. PMID: 30006315
-
Photoresponsive Chemistries for User-Directed Hydrogel Network Modulation to Investigate Cell-Matrix Interactions.Acc Chem Res. 2025 Jan 7;58(1):47-60. doi: 10.1021/acs.accounts.4c00548. Epub 2024 Dec 12. Acc Chem Res. 2025. PMID: 39665396 Review.
-
Dynamic Hydrogels with Viscoelasticity and Tunable Stiffness for the Regulation of Cell Behavior and Fate.Materials (Basel). 2023 Jul 21;16(14):5161. doi: 10.3390/ma16145161. Materials (Basel). 2023. PMID: 37512435 Free PMC article. Review.
Cited by
-
Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels.J Mater Chem B. 2020 Sep 21;8(35):7835-7855. doi: 10.1039/d0tb01429j. Epub 2020 Jul 21. J Mater Chem B. 2020. PMID: 32692329 Free PMC article. Review.
-
Engineered hydrogels for mechanobiology.Nat Rev Methods Primers. 2022 Dec 15;2:98. doi: 10.1038/s43586-022-00179-7. Nat Rev Methods Primers. 2022. PMID: 37461429 Free PMC article.
-
Chemical Approaches to Dynamically Modulate the Properties of Synthetic Matrices.ACS Macro Lett. 2019 Jan 15;8(1):7-16. doi: 10.1021/acsmacrolett.8b00808. Epub 2018 Dec 22. ACS Macro Lett. 2019. PMID: 32405440 Free PMC article.
-
Dynamic Click Hydrogels for Xeno-Free Culture of Induced Pluripotent Stem Cells.Adv Biosyst. 2020 Nov;4(11):e2000129. doi: 10.1002/adbi.202000129. Epub 2020 Sep 13. Adv Biosyst. 2020. PMID: 32924337 Free PMC article.
-
Stimuli-Responsive Protein Hydrogels: Their Design, Properties, and Biomedical Applications.Polymers (Basel). 2023 Dec 8;15(24):4652. doi: 10.3390/polym15244652. Polymers (Basel). 2023. PMID: 38139904 Free PMC article. Review.
References
-
- Macri-Pellizzeri L, Pelacho B, Sancho A, Iglesias-Garcia O, Simon-Yarza AM, Soriano-Navarro M, Gonzalez-Granero S, Garcia-Verdugo JM, De-Juan-Pardo EM, Prosper F, Substrate stiffness and composition specifically direct differentiation of induced pluripotent stem cells, Tissue Eng Part A 21(9–10) (2015) 1633–41. - PubMed
-
- Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ, Huynh J, Mencia-Trinchant N, Negron Abril YL, Hassane DC, Bonassar LJ, Butcher JT, Weiss RS, Reinhart-King CA, Matrix stiffening promotes a tumor vasculature phenotype, Proc Natl Acad Sci U S A 114(3) (2017) 492–497. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

